4. Non-innocence of ruthenium coordinated "NO" and facile photocleavage of Ru-NO+/" bond:
The relevance of nitric oxide in biological and environmental processes and in particular its applications in the form of ruthenium nitrosyls as antitumor and antiseptic agents, have introduced a renewed interest. The non-innocent behavior of coordinated NO makes it susceptible to shuttle between NO+, NO" and NO , depending on the ancillary functionalities. In this context, a facile methodology towards the synthesis of {Ru-NO+}/ {Ru-NO"}/{Ru-NO } has been introduced via the judicious selection of ancillary ligands. Mechanistic details of the reactivity of M-NO+/" with the nucleophiles have also been established. The designed {Ru-NO+} and {Ru-NO"} undergo facile photogenaration of "NO" at different rates which could also be scavenged by the biological target, myoglobin or cytochrome-c (Scheme 4), extending an alternate basis for the metal complex derived suitable "NO" donors under biological conditions.
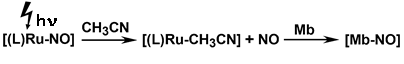
Representative references:
- Lahiri et al. Inorg. Chem., 2019, 58, 1627
- Lahiri et al. Angew. Chem. Int. Ed., 2009, 48, 424
- Lahiri et al. Inorg. Chem., 2008, 47, 3218