5. Development of Nano-Structured Heterogeneous as well as Homogeneous (Green) Catalysts for Hydrogenation and Oxygenation Processes:
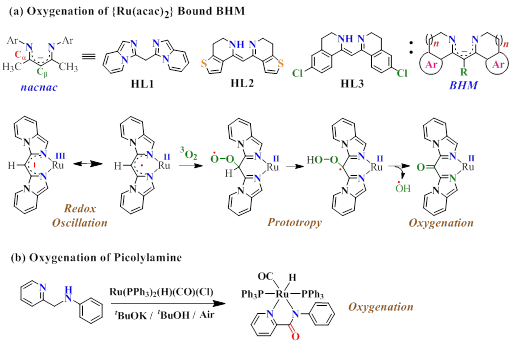
Dioxygen plays a crucial role in numerous biological processes. Most of these processes rely on the oxidizing power of dioxygen, where dioxygen undergoes reductive activation. However, triplet ground state of molecular oxygen (3 g ) provides a substantial kinetic barrier for its eduction due to high negative one-electron reduction potential (?0.33 V vs NHE in water at pH 7, 25 °C) and thus disfavors the auto-oxidation of diamagnetic organic molecules. Nature however overcomes the spin state barrier by employing transition metal ions that exist in spin-open ground states, which can react directly with the triplet dioxygen to facilitate the reduction of O2. Our basic interest in this regard is to design transition metal complexes which have accessible multiple redox states including a number of open-shell states that can activate dioxygen. Thus, oxygenation scenario of bis(heterocyclo)methanide (BHM), a superior analogue of ubiquitous -diketiminate,and picolylamine analogues upon ruthenium chelation has been scrutinized. Accessibility of multiple redox steps of ruthenium facilitates resonance assisted redox tuning at the metal-ligand interface (MpLn Mp+1Ln?1) which in effect facilitates the desired oxygenation process (Scheme 5).
Representative references:
- Lahiri et al. Inorg. Chem., 2020, 59, 1355
- Lahiri et al. Inorg. Chem., 2019, 58, 11410
- Lahiri et al. Inorg. Chem., 2017, 56, 14900