2. Delicate electronic structural aspects of redox non-innocent molecular systems :
Quinones, the naturally occuring redox-active molecules are widely distributed, functioning in vital electron transport processes where they often interact with transition metal ions or exhibit specific toxicity. ortho-Quinone-containing prosthetic groups in metallo-quinoproteins are well known in the form of pyrrolo-quinoline-quinone (PQQ), tryptophan-tryptophyl-quinone (TTQ), topaquinone (TPQ), and lysine-tyrosyl-quinone (LTQ). Catechols as 2e /2H+-reduced o-quinones are being investigated as anti-oxidants (polyphenols), as neurotransmitters (catecholamines), and as precursors of melanin pigments. para-quinones such as vitamin K derivatives, ubiquinones or plastoquinones also play many important roles in energy conversion (photosynthesis, respiration) or information transfer. In order to rationalize the intricate electronic interactions between transition metal ions and quinone redox systems in biochemical environments there have been considerable efforts at the molecular level of metal complexes with quinonoid ligands. The remarkable mixing of metal and quinonoid frontier orbitals in the complex frameworks extends challenges in assigning their precise valence and spin states at the metal-ligand interface both in the native and accessible redox states (Scheme 2). Thus, the issue of sensitive electronic structural aspects of such redox non-innocent assemblies has been addressed using spectroscopic, structural, magnetic and theoretical calculations.
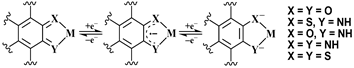
Scheme2
Furthermore, extensive attempts have also been made to explore the feasibity of non-innocent features of otherwise well established redox-inactive ligand frameworks (Scheme 3) i.e. fractional or hidden non-innocence on their coordiantion to selected metal fragments.
The detailed investigations in this regard have revealed the frequent involvement of resonating forms of feasible alternate electronic states including the complex redox induced electron transfer (RIET) situation along the accessible redox chains.
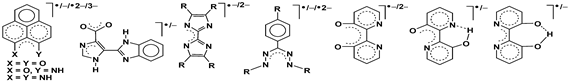
Scheme3
Representative references:
- Lahiri et al. Inorg. Chem., 2020, 59, 4397
- Lahiri et al. Chem. Eur. J., 2013, 19, 7384
- Lahiri et al. Chem. Eur. J., 2012, 18, 14434
- Lahiri et al. J. Am. Chem. Soc., 2008, 130, 3532
- Lahiri et al. Angew. Chem. Int. Ed., 2007, 46, 1778