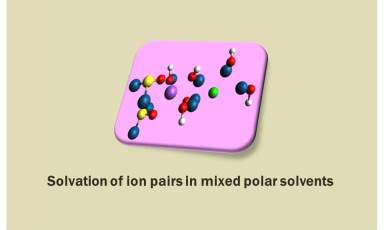
Our group performs molecular dynamics simulations of ion pairs in polar solvents such as water, dimethyl sulfoxide (DMSO), methanol (MeOH), and acetone and mixed polar solvents such as water/acetone, water/methanol, DMSO/MeOH, Water/DMSO. We study the solvation structure and preferential solvation of ion pairs in mixed polar solvents.
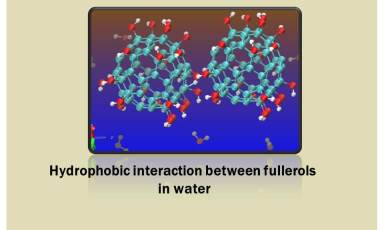
We also study the hydrophobic interactions in between fullerene derivatives, carbon-nanotubes in mixed polar solvents. We investigate the effect of osmolytes (urea, taurine etc.) on hydrophobic interactions between hydrophobes (methane, neopentane etc.).
We examine the rate of solvolysis and rate of dissociation of exo and endo norbornyl derivatives in pure (water, MeOH, acetone, DMSO etc.) mixed polar solvents (water/DMSO, water/acetone and water-methanol etc.).
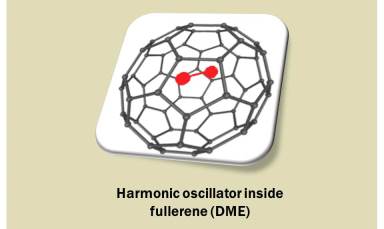
We investigate the effect of solvents on stretching frequency of harmonic oscillator in mixed polar solvents with the help of Density Matrix Evolution (DME).
We calculate the Franck-Condon factors for diatomic molecules with the help of Furrier Grid Hamiltonian Method (FGH).
We use our own Molecular dynamics code and gromacs package for classical molecular dynamics simulations. We have our own code for DME method and FGH method.