Glycochemistry Research Lab
Department of Chemistry
Indian Institute of Technology Bombay
Powai, Mumbai 400076
Phone : 022-2576 7166
Fax : 022 -2576 7152
Email : suvarn[at]chem.iitb.ac.in
Synthesis of bacterial glycans :
Bacteria have unusual glycans on their surfaces which distinguish them from the host cells. These unique structures offer avenues for targeting bacteria with specific therapeutics and vaccines. However, the rare sugars are not accessible in acceptable purity and amounts by isolation. Availability of orthogonally protected monosaccharide building blocks through efficient chemical synthesis is a crucial step toward the development of glycoconjugate vaccines. Towards this goal, we have established a general and divergent strategy for the synthesis of the bacterial deoxy amino hexopyranoside and D-glycosamine building blocks from D-mannose via double serial and double parallel displacements of 2,4-bis-triflates by azide, phthalimide, nitrite and acetate as nucleophiles.The methodology is applied to the first total synthesis of the L-serine linked trisaccharide of Neisseria meningitides, and synthesis of a rare disaccharide fragment of the zwitterionic polysaccharide A1 of Bacteroides fragilis and a selectively protected Tn antigen. The short and efficient protocol is expected to speed up bacterial glycan assembly and give a rapid access to the prokaryotic glycome. Synthesis of various bacterial glycoconjugates is underway.
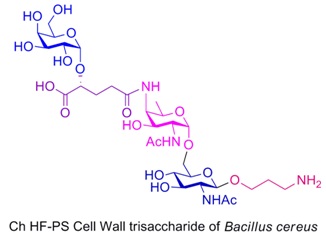
The same methodology is applied for the first total synthesis of Ch HF-PS, a cell wall trisaccharide repeating unit of B. Cereus. The synthetic trisaccharide is appended with an aminopropyl linker at the reducing end to allow for conjugation to proteins and microarrays. The convergent synthesis involves transformation of D-mannose into an orthogonally protected rare AAT sugar building block, two consecutive α- stereoselective glycosylations, β-selective attachment of the linker by solvent participation, and amide bond formation, as key steps.
Publications :
- Clark, E. L.; Emmadi, M.; Krupp, K. L.; Podilapu, A. R.; Helble, J. D.; Kulkarni, S. S.; Dube, D. H. ACS Chem. Biol. 2016, 11, 3365-3373. DOI :
- Sanapala, S. R.; Kulkarni, S. S. Org. Lett. 2016, 18, 3790-3793. DOI :
- Sanapala, S. R.; Kulkarni, S. S. J. Am. Chem. Soc. 2016, 138, 4938-4947. DOI :
- Sanapala, S. R.; Kulkarni, S. S.; RSC Adv. 5, 22426-22430, 2015 . DOI :
- Jana, S., Emmadi, M., Kulkarni, S. S., Isr. J. Chem. 55, 398-402. DOI :
- Behera, A.; Emmadi, M.; Kulkarni, S. S., RSC Advances, 2014, 4, 58573-58580. DOI :