Publications
Year Index :
2024 | 2023 |2021 | 2020 | 2019 | 2018 | 2017 | 2016 | 2015 | 2014 | 2013 | 2012 | 2011 | 2010 | 2009 | 2008 | 2007 | 2006 | 2005 | 2004 | 2003 |
2024
2023
2021
2020
2024 | 2023 |2021 | 2020 | 2019 | 2018 | 2017 | 2016 | 2015 | 2014 | 2013 | 2012 | 2011 | 2010 | 2009 | 2008 | 2007 | 2006 | 2005 | 2004 | 2003 |
2024
2024
-
88. "Thapsigargin: a promising natural product with diverse medicinal potential - a review of synthetic approaches and total syntheses"- Anisha Suresh, Dibyojeet Bagchi and Krishna P. Kaliappan, Org. Biomol. Chem., 2024,DOI:

Thapsigargin, a sesquiterpene lactone, naturally occurring in the roots and fruits of the Mediterranean shrub Thapsia garganica L, is known to the practitioners of traditional medicines since the medieval ages as a cure for rheumatic pain, lung diseases, and female infertility. This naturally occurring guaianolide has shown remarkable activity for Sarco endoplasmic reticulum Ca2+ ATPase inhibition, which eventually renders it fit as a potential candidate for anti-cancer drugs. Mipsagargin, a prodrug derived from thapsigargin, is under clinical trials for the treatment of glioblastoma. Recently, thapsigargin has shown promise as an antiviral against SARS-CoV-2. Limited natural availability and challenging synthesis have prompted research into new synthetic pathways. This review discusses significant synthetic approaches and total syntheses of thapsigargin reported to date.
87. "A relay ring-closing metathesis/Diels-Alder approach to sugar-derived pluramycin-hybrids" - Ajad Singh and Krishna P. Kaliappan, Org. Biomol. Chem., 2024, 22, 6727-6741. DOI:
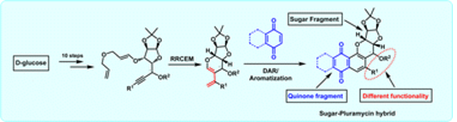
Herein, we present a general approach for synthesizing pluramycin hybrids, which are analogous to the pluramycinone carbocyclic skeleton. This method involves a sequence of relay ring-closing enyne metathesis, Diels-Alder and oxidative aromatization reactions to synthesize pluramycinone-sugar hybrids. As part of our ongoing research, we have successfully synthesized two pluramycin hybrid analogues by carefully monitoring the late-stage oxidative aromatization steps, which depend on the stereo-orientation of the Diels-Alder cycloadduct at the C-4 center. The undesired ring-opening product can also serve as a C-glycoside analog, providing a versatile convergent route to access both types of hybrids and highlighting the significance of this strategy.
-
86. "Synthesis of N-Alkyl Substituted Benzimidazoquinazolinones" - Gaurav G. Dake and Krishna P. Kaliappan, ACS Omega 2024, 9, 31, 33805-33814. DOI:
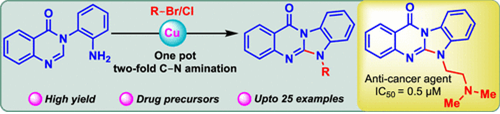
Aromatic N-heterocycles, especially benzimidazoquinazolinones featuring alkyl chains, hold significant pharmaceutical relevance. Here, we introduce a streamlined one-pot, 2-fold Cu-catalyzed C-N bond formation protocol for the efficient synthesis of diverse N-alkyl benzimidazoquinazolinone derivatives. This method showcases a broad substrate scope, leveraging readily accessible alkyl halides and delivers the desired cyclized products in excellent yields. Additionally, the methodology enabled the synthesis of an antitumor agent with satisfactory yield, highlighting its utility in medicinal chemistry endeavors.
-
85. "Asymmetric Total Synthesis of 4-Hydroxy-8-O-methyltetrangomycin, 4-Hydroxytetrangomycin, and 4-Keto-8-O-methyltetrangomycin" - Ajad Singh and Krishna P. Kaliappan, J. Org. Chem. 2024, 89, 15, 10965-10973 DOI:
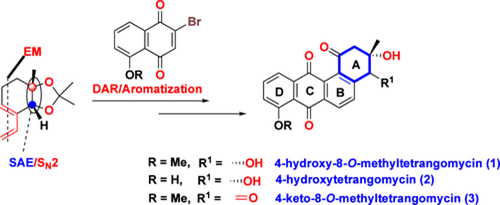
Herein, we report the first asymmetric total synthesis of 4-hydroxy-8-O-methyltetrangomycin, 4-hydroxytetrangomycin, and 4-keto-8-O-methyltetrangomycin, angucyclinones featuring a highly oxidized nonaromatic A ring. A sequential enyne metathesis/Diels-Alder approach was utilized successfully to construct the tetracyclic skeleton of the angucyclinones. Late-stage acetonide deprotection challenges were overcome by A ring functional group manipulation, yielding a dihydroxy intermediate prior to the benzylic photo-oxidation, facilitating the total syntheses of angucyclinones. The key stereocenter was established through a known Sharpless asymmetric epoxidation/regioselective epoxide opening reaction.
-
84. "Total Syntheses of Discoipyrroles A, B, and C, Three Marine Natural Products" - Gaurav G. Dake and Krishna P. Kaliappan, J.Org. Chem., 2024, 89, 8, 5825-5832. DOI :
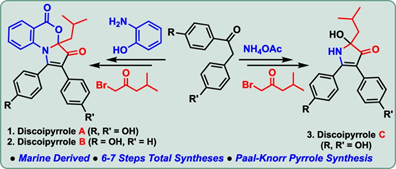
Herein, we describe our efforts culminating in the total syntheses of discoipyrroles A, B, and C in 6, 6, and 7 steps respectively with excellent overall yield. Total syntheses of these unique natural products have been accomplished involving microwave-mediated Paal-Knorr pyrrole synthesis, Pd-catalyzed carbonylative transformation, and MoOPH (Vedejs reagent) oxidation as key reactions to construct the 1,2,3,5-tetrasubstituted pyrrole and oxidative cyclization of highly substituted pyrrole as key steps.
2023
2023
-
83. "Divergent Synthesis of 6- or 7-Aza-Indazoles via Intramolecular Diels-Alder Cascade of Pyrazines" - Nicolas Brach, Lucas Popek, Mathieu Truong, Claire Laurent, Vincent Bizet, Krishna P. Kaliappan, and Nicolas Blanchard, Org. Lett., 2023, 25, , 43, 7847-7851. DOI :
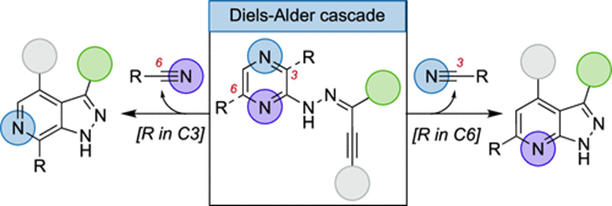
Pyrazines are reactive 4π partners in intermolecular Diels-Alder cycloaddition with exceptionally activated dienophiles or in an intermolecular version at elevated temperatures. Herein, it is shown that an intramolecular cascade could occur even at room temperature, delivering a collection of 6- or 7-aza-indazoles. An interesting substituent effect of the cycloaddition precursor on the product distribution was uncovered, and in situ NMR studies were conducted to gain insights into this unexpected selectivity.
2021
2021
-
82. "A Serendipitous One-Pot Cyanation/Hydrolysis/Enamide Formation: Direct Access to 3-Methyleneisoindolin-1-one" - Trisha Banik and Krishna P. Kaliappan, Chem. Eur. J., 2021, 27, 628-633 DOI :
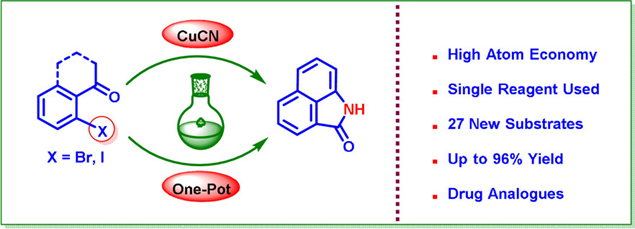
A direct, one-pot conversion of 2’-haloacetophenones to 3-methyleneisoindolin-1-one scaffolds using CuCN as the sole reagent without the need for moisture-free or anaerobic conditions is reported. This serendipitously discovered transformation with a broad substrate scope provides a significantly different route towards these important scaffolds. The scope of the method has also been further extended towards the synthesis of three special scaffolds, which are analogous to various bio-active drugs.
2020
2020
-
81. "Synthetic studies on palmerolide C: synthesis of an advanced intermediate towards the revised structure of palmerolide C" - Ashik A. Sayyad, Khushboo Kaim and Krishna P. Kaliappan, Org. Biomol. Chem., 2020, 18, 5937-5950 DOI :
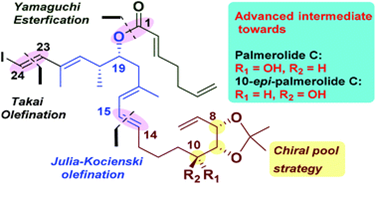
A stereoselective synthesis of the highly advanced intermediates towards the revised structure of palmerolide C and 10-epi-palmerolide C is described in this paper. The required key fragments C1-C6, C7-C14 and C15-C23 have been successfully assembled in a convergent manner to access the C1-C23 framework bearing all the five stereocenters present in the natural product. The synthesis involves the Julia-Kocienski reaction, Yamaguchi esterification, Takai olefination and regioselective epoxide opening as key steps. The proposed route is flexible and could also be applied to the synthesis of structurally related palmerolides.
80. "Synthesis of C9-C13 and C15-C21 Subunits of Discodermolide". - Debjani Si and Krishna P. Kaliappan, Asian J. Org. Chem. , 2020, 9, 1205-1212 . DOI :
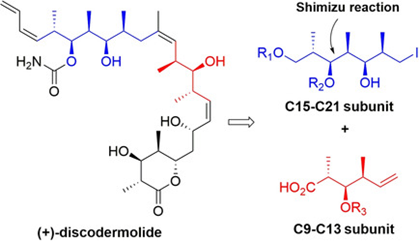
A new synthetic strategy for the construction of two polypropionate subunits of discodermolide, a highly potent anticancer natural product, is reported. The strategy relies on two key reactions: a Shimizu non-aldol reaction, a stereoselective hydrogenolysis of alkenyl epoxides, to generate the syn hydroxy-methyl moiety and a NaBH4-BF3.OEt2 mediated hydride addition reaction. By utilizing this strategy, two different fragments of discodermolide, C9-C13 subunit and C15-C21 subunit have been successfully synthesized.
79. "Synthesis of the C1-C10 Fragment of Muamvatin". - Anandaraju Bandaru and Krishna P. Kaliappan, Chem. Asian J., 15, 2208-2211 DOI :
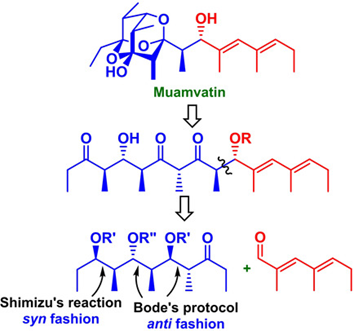
This report delineates our efforts towards the synthesis of a stereochemically well-defined ketone, C1-C10 fragment of muamvatin, the first example of 2, 4, 6-trioxaadamantane ring skeletal polypropionate marine natural product, using two non-aldol variants. i) Shimizu reaction, a Pd(0) mediated stereoselective epoxy-ring opening of alkenyl oxiranes, was employed for the stereoselective installation of methyl groups in syn-fashion and ii) Bode's protocal, a NHC-mediated reaction on β-epoxy aldehydes, was utilized for stereoselective construction of methyl and hydroxyl groups in anti-fashion.
78. "Synthesis of C1-C9 and C10-C21 Fragments of (-)-Dolabriferol". - Anandaraju Bandaru, Debjani Si and Krishna P. Kaliappan, Asian J. Org. Chem.,, 2020, 9, 1045-1052 . DOI :
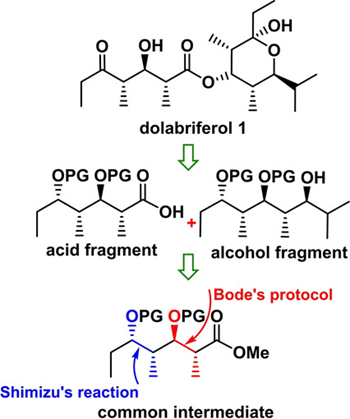
Our efforts on the synthesis of C1-C9 and C10-C21 subunits of dolabriferol, a non-contiguous polypropionate marine natural product, from a common intermediate using two non-aldol variants is reported. i) Shimizu reaction, a Pd(0) mediated stereoselective epoxy-ring opening of alkenyl oxiranes, has been employed for the stereoselective installation of methyl groups at C4 and C13 and ii) Bode's protocol, a NHC-mediated reaction on ?-epoxy aldehydes, has been utilized for stereoselective construction of methyl and hydroxyl groups in anti-fashion at C6-C7 and C15-C16 in the natural product.
77. "A One-Pot Copper-Catalyzed 3-Fold C-N Bond Coupling Strategy to the Synthesis of Substituted Benzimidazoles". - Parthasarathi Subramanian and Krishna P. Kaliappan, Eur. J. Org. Chem,, 2020, 44, 6915-6921 . DOI :
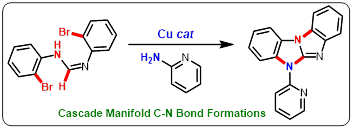
One-pot reaction leading through directly accessing biologically relevant heterocycles is highly recommended in chemical synthesis. One such standout method, manifold C-N bond formations route to the synthesis of benzimidazo[1,2-a]benzimidazoles is described. Our methodology involves a copper catalyzed intramolecular C-N coupling of N,N'-bis(2-bromophenyl)formimidamide to provide 1-(2-bromophenyl)-1H-benzimidazole which upon intermolecular coupling with 2-amino pyridines and a cascade sp2 C-H amination obtained a range of benzimidazo[1,2-a]benzimidazoles.
76. "Serendipitous Synthesis of Pyridoquinazolinones via an Oxidative C-C Bond Cleavage". - Matthias Brendel, Priyanka R. Sakhare, Gaurav Dahiya, Parthasarathi Subramanian and Krishna P. Kaliappan, J. Org. Chem., 2020, 85, 8102-8110. DOI:
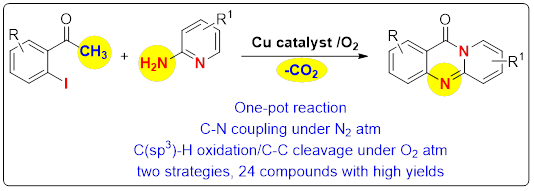
A direct one-pot copper catalyzed oxidative C-C bond cleavage route to the synthesis of pyridoquinazolinones is described. This one-pot strategy involves a copper catalyzed C-N coupling followed by concomitant C(sp3)-H oxidation, amidation via oxidative C-C bond cleavage under O2 atmosphere to deliver the target molecules in high yields.
75. "Synthetic approaches towards cortistatins: evolution and progress through its ages". - Satrajit Indu and Krishna P. Kaliappan, Org. Biomol. Chem., 2020, 18, 3965-3995. DOI:
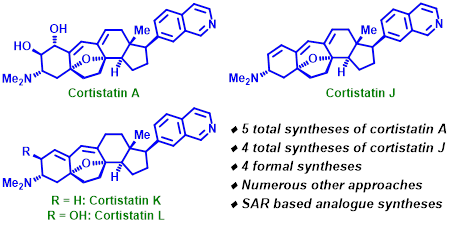
Cortistatins are a family of steroidal alkaloids with a unique pentacyclic skeleton, having immensely potent anti-angiogenetic activities. Given the scarcity in the natural availability of these compounds, their syntheses became major attractions in organic chemistry. Along with total synthesis of the most potent congeners in the family: cortistatins A and J, the synthesis of two other members have been successfully completed, while various other analogues have also been designed with variable degrees of biological activities. This review is an exhaustive coverage of the significant attempts towards constructing this highly challenging molecule and also aims to highlight the deep understanding of the structure–activity relationships of these compounds, which have been garnered over time.
74. "Construction of key building blocks towards the synthesis of cortistatins". - Satrajit Indu, Rahul D.Telore and Krishna P. Kaliappan, Org. Biomol. Chem., 2020, 18, 2432-2446. DOI:
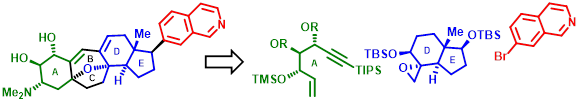
This work reports the construction of key building blocks towards the synthesis of cortistatins; a family of steroidal alkaloids. Cortistatin A, being a primary target due to its superior biological properties over other congeners, has been prepared by two different synthetic routes. Synthesis of the precursor to the heavily substituted A-ring starting from D-glucose and construction of the DE-ring junction employing a Hajos–Parrish ketone as a chiral pool have been demonstrated. Efforts are underway to assemble these key fragments and build towards the total synthesis of cortistatin A.
73. "A process for preparing 1,8-naphthalimide, biphenyl dicarboxamide and n-heterocyclic amides from 1, 2 diketones". - Krishna P. Kaliappan, Priyanka R. Sakhare and Parthasarathi Subramanian, Indian Pat. App. , 2020, IN 201821028477 A 20200207
2019
-
72. "Copper Catalyzed Oxidative C-C Bond Cleavage of 1,2-Diketones: A Divergent Approach to 1,8-Naphthalimides, Biphenyl-2,2?-dicarboxamides, and N-Heterocyclic Amides". - Priyanka R. Sakhare, Parthasarathi Subramanian, and Krishna P. Kaliappan, J. Org. Chem., 2019, 84, 2112-2125. DOI :
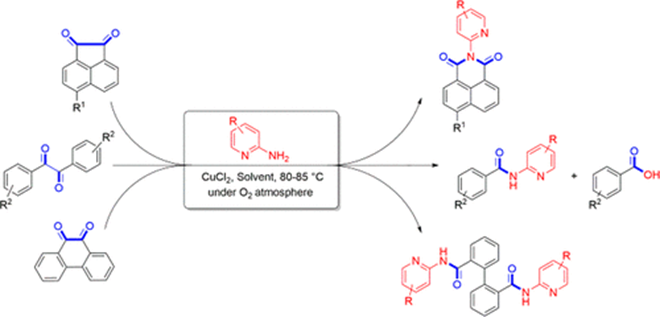
We report here a simple and efficient copper catalyzed oxidative C-C bond cleavage of stable aromatic cyclic-fused and acyclic 1,2-diketones to deliver amides and imides in high yields. This newly developed protocol provides an excellent tool to transform structurally different 1,2-diketones into different products under the same reaction conditions. The key synthetic features of this methodology are the formation of 1,8-naphthalimides and biphenyl-2,2'-dicarboxamide motifs in high yields. The fluorescent studies of 1,8-naphthalimide derivatives were also carried out in order to show the potential application of these scaffolds.
2018
-
71. "Transition-Metal-Free Multicomponent Approach to Sterioenriched Cyclopentyl-isoxazoles through C-C bond Cleavage". - Parthasarathi Subramanian, and Krishna P. Kaliappan, Chem. Asian J., 2018,13,2031-2039. DOI
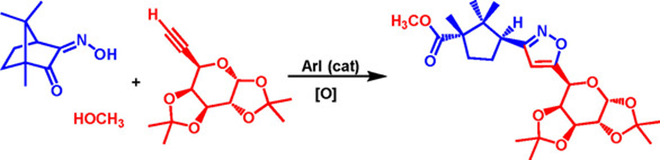
An efficient multicomponent reaction for the synthesis of stereoenriched cyclopentyl-isoxazoles from camphor-derived a-oximes, alkynes, and MeOH is reported. Our method involved a series of cascade transformations, including the in situ generation of an IIII catalyst, which catalyzed the addition of MeOH to a sterically hindered ketone. Oxidation of the oxime, and rearrangement of the a-hydroxyiminium ion generated a nitrile oxide in situ, which, upon [3+2] cycloaddition reaction with an alkyne, delivered the regioselective product. This reaction was very selective for the syn-oxime. This multicomponent approach was also extended to the synthesis of a new glycoconjugate, camphoric ester-isoxazole C-galactoside.
70. "Strategic innovations for the synthesis of vinigrol". - Vipul V.Betkekar and Krishna P. Kaliappan, Tetrahedron Lett., 2018, 59, 2485-2501. DOI :
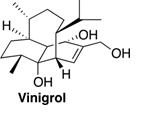
Since the isolation of unique tricyclic diterpenoid vinigrol, its promising biological activities and complex framework have resulted in numerous synthetic efforts to the total synthesis of this venerable molecule for the past two and half decades. This mini review highlights the significance of various synthetic strategies towards this structurally unique and challenging natural product.
69. "A new and informative [a,b,c,d] nomenclature for one-pot multistep transformations: a simple tool to measure synthetic efficiency." - Satrajit Indu and Krishna P. Kaliappan, RSC Adv., 2018, 8, 21292-21305. DOI :
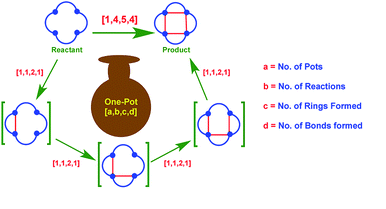
Domino, cascade and tandem reactions constitute the most efficient and creative chemical transformations with a huge domain of synthetic utility and applications. A number of reactions may be achieved in a single pot, accompanied by the formation of new rings and new bonds, leading towards higher molecular complexity. A lack of one unified, yet informative descriptor often understates the synthetic ingenuity of certain highly creative transformations. In this review, we propose a new tetra-coordinated [a,b,c,d] nomenclature which takes into account and displays the basic parameters which generally indicate the level of efficiency of a chemical transformation. An almost exhaustive set of one-pot multistep reactions may be described by this system and this review is an attempt to display the one-pot multistep transformations reported from our group and to classify them based on our proposed descriptor.
68. "A Unprecedented, Lewis Acid-Mediated, Metal-Free Iodoannulation Strategy to Aromatic Iodides". - Trisha Banik, Vipul V. Betkekar and Krishna P. Kaliappan, Chem. Asian J., 2018, 13, 3676-3680. DOI :
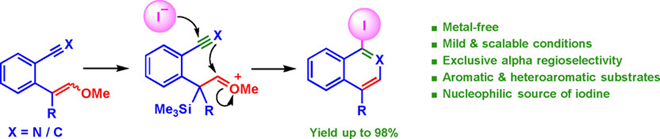
A direct transformation of ortho-alkynylated aromatic vinyl ethers to 1-iodonaphthalenes and other iodo-heterocycles under mild Lewis acidic conditions in the presence of iodide as an external nucleophile is reported. The first example of an iodoannulation strategy using a nucleophilic source of iodine, coupled with good to excellent yields, exclusive alpha regioselectivity and a broad substrate scope makes this work an attractive avenue towards the construction of aromatic iodides.
2017
-
67. "Sequential Enyne?Metathesis/Diels-Alder Strategy: Rapid Access to Sugar-Oxasteroid-Quinone Hybrids". - Ashik A. Sayyad and Krishna P. Kaliappan, Eur. J. Org. Chem., 2017, 34, 5055-5065. DOI :
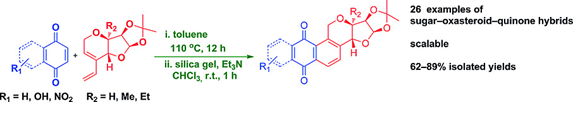
A sequential enyne-metathesis/Diels–Alder strategy is reported for the synthesis of a new class of sugar–oxasteroid–quinone hybrid molecules. 1,2:5,6-Di-O-isopropylidine-D-glucose was chosen as a chiral-pool starting material. Various sugar-derived enynes were synthesised from the common starting material. Dienes derived from these enynes were treated with various quinone dienophiles under Diels–Alder reaction conditions to give a library of sugar–oxasteroid–quinone hybrids.
2016
-
66. "Recent Trends in Copper-Catalyzed C-H Amination Routes to Biologically Important Nitrogen Scaffolds". Parthasarathi Subramanian, Georg C. Rudolf, and Krishna P. Kaliappan, Chem.Asian.J., 2016, 11, 168-192. DOI :
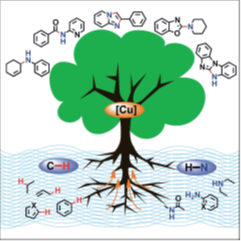
Nitrogen-containing heterocycles have found remarkable applications in natural product research, material sciences, and pharmaceuticals. Although the synthesis of this interesting class of compounds attracted the interest of generations of organic chemists, simple and straightforward assembly methods based on transition-metal catalysis have regularly been elusive. The recent advancements in the development of C−H functionalization have helped in accomplishing the synthesis of a variety of complex heterocycles from simple precursors. This Focus Review summarizes the recent advances in one particular field: the copper-catalyzed C−N bond formation reactions via C−H bond functionalization to furnish a comprehensive range of nitrogen heterocycles. Applicability and synthetic feasibility of a particular reaction represent major requirements for the inclusion in this review.
65. "A Copper Catalyzed Cascade Amination Route to N-Aryl Benzimidazoquinazolinones". Arpan Banerjee, Parthasarathi Subramanian and Krishna P. Kaliappan. J.Org.Chem., 2016, 81, 10424-10432. DOI :
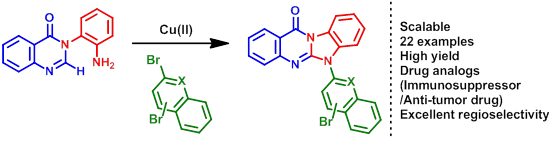
An efficient one-pot Cu-catalyzed C-H functionalization/two-fold C-N bond formation protocol for the syntheses of N-aryl benzimidazoquinazolinones is being reported. This strategy involves a Cu-catalyzed C-N bond coupling reaction between N-anilinoquinazolinones and aryl/heteroaryl halides followed by acetate ligand assisted intramolecular C-H amination. This reaction is general and straightforward for the synthesis of anti-cancer drug analogs of benzimidazoquinazolinones.
2015
-
64. "An enantioselective total synthesis of Sch-725674". Kota Ramakrishna and Krishna P. Kaliappan, Org. Biomol. Chem., 2015, 13, 234-240. DOI
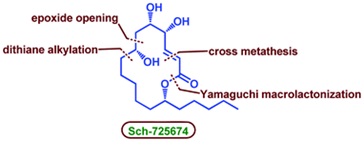
An enantioselective total synthesis of Sch-725674, a unique 14-membered macrolactone, has been accomplished in 13 steps. The step-wise dithiane alkylation served as a strategic step to assemble the upper and lower fragments of the molecule, whereas cross metathesis reaction, Yamaguchi macro-lactonization and a substrate controlled stereoselective reduction are used as key steps to complete the total synthesis.
2014
-
63. "A One-Pot Copper Catalyzed Biomimetic Route to N-Heterocyclic Amides from Methyl Ketones via Oxidative C-C Bond Cleavage". Parthasarathi Subramanian, Satrajit Indu and Krishna P. Kaliappan, Org. Lett., 2014, 16 (23), 6212-6215.
DOI
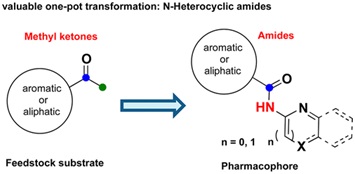
A direct one-pot Cu-catalyzed biomimetic oxidation of methyl ketones to pharmaceutically important N-heterocyclic amides is reported. The scope of the method is broad, scalable, and mild, and the reaction is tolerant with various acid, base sensitive functionalities with multiple heteroatoms and aryl halides. The extensive mechanistic studies suggest that this reaction follows the Luciferin-Luciferase-like pathway.
62. "A Domino Enyne/IMDA Approach to the Core Structure of (-) Vinigrol". Vipul V. Betkekar, Ashik A. Sayyad and Krishna P. Kaliappan, Org. Lett., 2014, 16(21), 5540-5543 DOI :
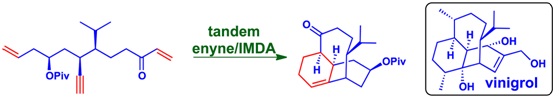
We report here an enantioselective formal synthesis of vinigrol involving a 1-2-3 strategy: one pot and tworeactions with the formation of three rings leading to the core structure of vinigrol from its stereochemically well-defined acyclic precursor.
61. "Transition Metal Catalyzed Selective Cyclization Strategy to 2-Substituted Benzofurans and Indoles En-Route to the Oxa-Analogs of Isocrytolepine". Satrajit Indu, Parthasrathi Subramanian and Krishna P. Kaliappan, Eur. J. Org. Chem., 2014,7193-7202. DOI :
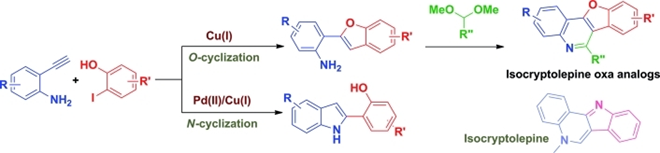
A selective catalytic route to benzofurans and indoles from similar starting materials has been developed. A two-step protocol that involves a transition-metal-catalyzed domino Sonogashira coupling between 2-ethynylanilines and 2-iodophenols followed by selective O- and N-cyclizations afforded 2-(benzofuran-2-yl)anilines and 2-(indol-2-yl)phenols, respectively. The 2-(benzofuran-2-yl)anilines were further utilized in a Lewis acid catalyzed Pictet–Spengler-type cyclization to prepare the oxa analogues of isocryptolepine.
60. "A Unified Strategy Towards N-Aryl Heterocycles by a One-Pot Copper-Catalyzed Oxidative C-H Amination of Azoles". Parthasarathi Subramanian and Krishna P. Kaliappan, Eur. J. Org. Chem., 2014, 5986-5997. DOI:
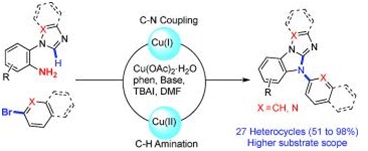
An efficient one-pot synthesis of N-aryl-substituted heterocycles by a Cu-catalyzed two-fold C-N bond formation is reported. This strategy involves a CuI-catalyzed C-N bond-forming reaction between azoles and electron-deficient bromopyridines followed by an intramolecular sp2 C-H amination. One of the products thus formed has been successfully used as a ligand for the synthesis of a Pd complex.
59. "Total syntheses of rubiginone A2, C2, andfujianmycin A". Devendar G. Vanga and Krishna P. Kaliappan, RSC Adv., 2014, 4, 12716-12722. DOI :
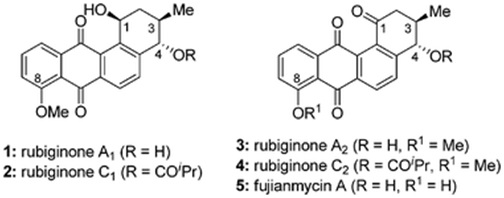
The total syntheses of rubiginone A2, C2 and fujianmycinA are described. The synthesis involves Diels-Alder/aromatization and photo chemical reactions as key steps to construct the tetracyclic frame ofbenz[a]anthraquinone skeleton. Sharplessepoxidation, a copper-catalyzed regioselective epoxideopening, and enyne metathesis reactions are utilized as the key steps for the synthesis of chiral vinylcyclohexene.
2013
2013
58. "Kinugasa Reaction: A Direct One Pot Route to Highly Functionalized β-Lactams." Rama K. Khangarot and Krishna P. Kaliappan, Eur. J. Org. Chem., 2013, 13,7664-7677. DOI.

The β-lactam antibiotics are among the most commonly prescribed drugs in the world and their importance has been demonstrated by the isolation and syntheses of several classes of these agents. Of the synthetic routes used to access this interesting scaffold, the Kinugasa reaction utilizes a convergent strategy based on cycloaddition between readily available terminal alkynes and nitrones. Asymmetric versions involving chiral catalysts, chiral auxiliaries or chiral substrates have also been reported. This article gives a brief overview of the Kinugasa reaction and of recent advances since its discovery.
57. "A hybrid approach to new molecular scaffolds" Kalanidhi Palanichamy and Krishna P. Kaliappan, Pure Appl. Chem., 2013, 85, 1185-1202. DOI :
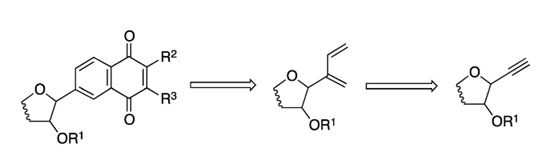
Our various efforts toward the synthesis of a set of novel sugar hybrid scaffolds of several biologically active natural products such as taxol, steroids, ?-lactams, and otteliones are presented. We have shown the application of the hybrid approach to design and rapidly generate a library of novel natural product-like compounds, which may have interesting biological features, using metathesis and/or cycloaddition reactions as key steps.
56. "A Stereoselective Synthesis of Trifluoromethyl-Analogues of Polyhydroxy Pyrrolidines" Rama K. Khangarot and Krishna P. Kaliappan, Eur. J. Org. Chem., 2013, 13, 2692-2698. DOI :
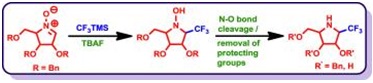
Incorporation of fluorine atoms into organic molecules significantly enhances many of their properties, such as solubility, metabolic stability, and bioavailability. Among organofluorine molecules, trifluoromethylated compounds play a unique and important role in agricultural and medicinal chemistry. An efficient strategy for the synthesis of a variety of trifluoromethylated polyhydroxypyrrolidines is described. This strategy involves a diastereoselective nucleophilic addition reaciton of trimethyl(trifluoromethyl)silane to sugar-derived cyclic nitrones followed by reductive N–O bond cleavage and removal of benzyl groups.
55. "A One-Pot, Copper-Catalyzed Cascade Route to 2-Indolyl-C-glycosides" Parthasarathi Subramanian and Krishna P. Kaliappan, Eur.J. Org. Chem., 2013, 3, 595-604. (Highlighted in CHEMISTRYVIEWS on 06 Dec 2012). DOI :
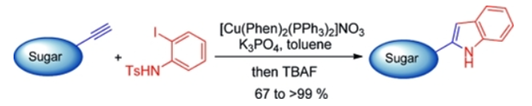
An efficient and high-yielding, one-pot, Cu-catalyzed synthesis of 2-indolyl-C-glycosides is delineated. The sequence involves a cascade Sonogashira type coupling and a hydroamination reaction between sugar-derived alkynes and N-tosyl-o-iodoaniline followed by the removal of the N-tosyl group to provide a library of 2-indolyl-C-glycosides in moderate to excellent yields.
2012
2012
54. "Recent Developments on Domino Metathesis Reactions in India" Rahul S. Nandurdikar and Krishna P. Kaliappan, CHIMIA 2012, 66, 892-896. DOI :
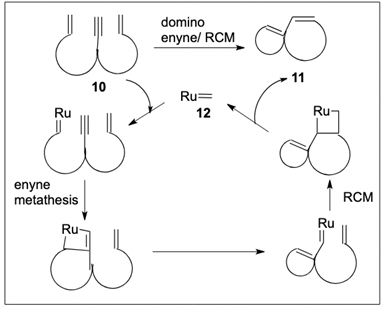
This review summarizes research work carried out in India on domino metathesis reactions using ruthenium-carbene complexes. This reaction has been widely used by synthetic chemists in India for the synthesis of polycyclic systems and complex molecular architectures.
53. "Total Synthesis and Stereochemical Assignment of (-)-Zenkequinone B" Vanga Devendar Goud and Krishna P. Kaliappan, Synlett 2012, 23, 2931-2934. DOI :
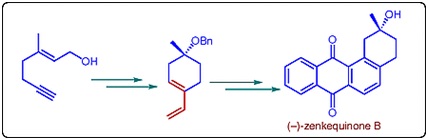
The first enantioselective total synthesis and a concise racemic synthesis of zenkequinone B are reported here by utilizing a sequential enyne metathesis, Diels-Alder and aromatization reactions.
52. "A Concise Total Synthesis of (+)-Cladospolide D" Debjani Si and Krishna P. Kaliappan Synlett 2012, 23, 2822-2826. DOI :
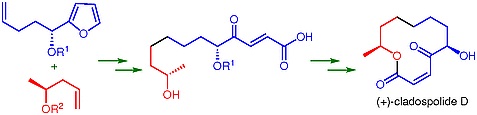
A short and convergent total synthesis of (+)-cladospolide D is delineated, which involves olefin cross metathesis andfuran oxidation to access the γ-oxo-α,β-unsaturated acid and Yamaguchi lactonization to construct the 12-membered ring as key steps.
51. "A Stereoselective Route to Aza-C-aryl Glycosides from Arynes and Chiral Nitrones" Rama K. Khangarot and Krishna P. Kaliappan, Eur. J. Org. Chem., 2012, 29, 5844-5854. DOI :
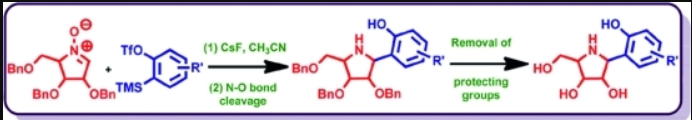
Arynes are regarded as potential and versatile intermediates in organic synthesis. These highly-reactive, strained and kinetically unstable species have numerous applications in synthetic chemistry. In this article, a highly-diasteroselective and efficient 1,3-dipolar cycloaddition reaction between a range of arynes and sugar-derived cyclic nitrones leading to an interesting class of sugar-based benzo[d]isoxazolines is described. These benzo[d]isoxazolines upon selective N–O bond cleavage provide various substituted aza-C-aryl glycosides in good yield. These substituted pyrrolidine derivatives are chiral aminophenols and could be potential chiral ligands and organocatalysts in asymmetric synthesis.
50. "Synthetic Utility of Sugar Derived Cyclic Nitrones: A Diastereoselective Synthesis of Linear 4-Azatriquinanes" Anandaraju Bandaru and Krishna P. Kaliappan, Synlett 2012, 23, 1473-1476. DOI :
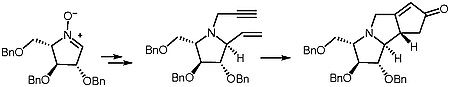
A diastereoselective Pauson-Khand reaction has been utilized as the key step in the construction of azatriquinanes from sugar-derived nitrones.
49. "An Efficient Copper Catalyzed 3-Component Synthesis of 3-C-Linked Glycosyl Iminocoumarins" Kalanidhi Palanichamy, Sankar R. Suravarupu, Krishna P. Kaliappan, Synthesis, 2012, 44, 1841-1848. DOI :
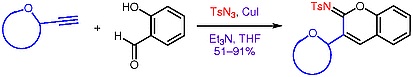
An efficient general strategy was developed for the synthesis of previously unknown 3-C-linked glycosyl iminocoumarins. The strategy involves a copper-catalyzed multicomponent reaction of sugar-derived alkynes with tosyl azide and salicylaldehyde to give a diverse array of glycosyl iminocoumarins in good yields. These compounds can serve as appropriate precursors for the synthesis of the corresponding coumarin 3-C-glycosides.
48. "A Tandem Enyne/Ring Closing Metathesis Approach to 4-Methylene-2-cyclohexenols: An Efficient Entry to Otteliones and Loloanolides" Vipul V. Betkekar, Samaresh Panda and Krishna P. Kaliappan, Org. Lett., 2012, 14, 198-201. DOI :
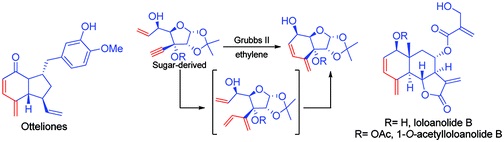
A short and efficient approach to a 4-methylene-2-cyclohexenone substructure present in otteliones and loloanolides is described. This strategy involves a tandem enyne/ring closing metathesis as the key reaction to construct this labile core unit.
47. "An Iterative Shimizu Non-aldol Approach for the Stereoselective Synthesis of C13-C22 Fragment of Callystatin A" Sandip A. Pujari and Krishna P. Kaliappan, Org. Biomol. Chem., 2012, 10, 1750-1753. DOI :
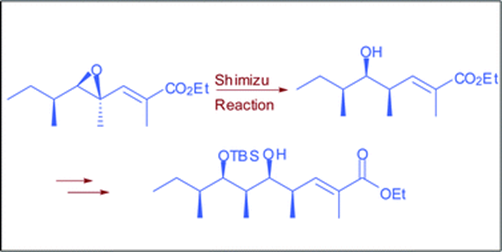
An efficient synthesis of the polypropionate framework of callystatin A has been achieved by utilizing the Shimizu reaction in an iterative fashion.
46. "A Unified Strategy for the Syntheses of Angucyclinone Antibiotics: Total Syntheses of Tetrangulol, Kanglemycin M, X-14881-E and Anhydrolandomycinone" Vanga Devendar Goud and Krishna P. Kaliappan, Eur.J. Org. Chem., 2012, 11, 2250-2259 DOI :
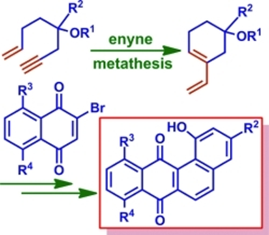
A unified strategy for the syntheses of angucyclinone antibiotics was developed utilizing sequential intramolecular enyne metathesis, Diels–Alder/aromatization, photooxygenation, and one-pot elimination/aromatization reactions. The diversity in this sequence was introduced in the Diels–Alder reaction where a common diene was treated with various appropriately functionalized quinones as the dienophiles to accomplish the total syntheses of tetrangulol, kanglemycin M, X-14881-E, and anhydrolandomycinone in moderate to good overall yields. The requisite diene was synthesized in excellent yield from a known enyne through an intramolecular enyne metathesis. The scope of this flexible and divergent strategy can be extended to the syntheses of similar scaffolds and unnatural aromatic angucyclinones.
2011
-
45. "A One Pot Deprotection and Intramolecular Oxa Michael Addition to Access Angular Trioxatriquinans".
K. Ramakrishna and Krishna P. Kaliappan, Synlett 2011, 17, 2580-2584. DOI :
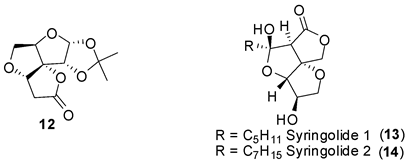
An efficient one-pot deprotection-oxa-Michael addition strategy has been used to synthesize a few trioxatriquinanes starting from commercially available sugars.
44. "A Radical Approach to Formal Total Syntheses of Platencin". K. Palanichamy A. V. Subrahmanyam and Krishna P. Kaliappan, Org. Biomol. Chem. 2011, 9, 7877-7886. DOI:
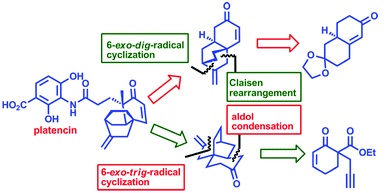
Two different strategies leading to formal total syntheses of platencin are described. The first strategy involving Claisen rearrangement and radical cyclization provides a rapid access to the core structure of platencin, and also use minimum protective-group operations. The second strategy, a protecting group-free route, utilizes a 6-exo-trig radical cyclization and aldol condensation as key steps leading to the formal synthesis of platencin.
43. "A Shimizu Aldol Approach to Formal Total Synthesis of Palmerolide A." S. A. Pujari, P. Gowrisankar and Krishna P. Kaliappan, Chem. Asian. J. 2011, 6, 3137-3151. DOI :
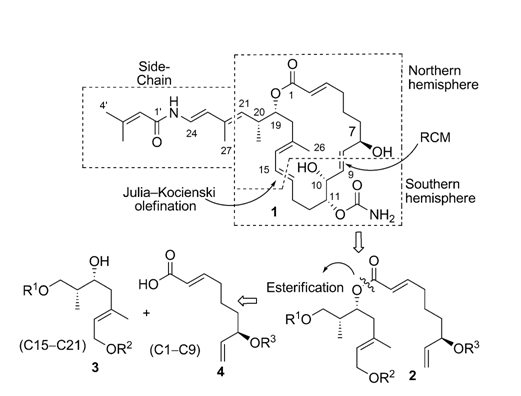
A formal total synthesis of palmerolide A has been accomplished by assembling three fragments by means of successive Julia–Kocienski olefination, Yamaguchi esterification, and ring-closing metathesis (RCM). Our initial efforts to combine the first two fragments through a Julia–Kocienski reaction between a secondary sulfone and a ketone were not successful; nevertheless, it was feasible between a primary sulfone and aldehyde. Yamaguchi esterification with the third fragment then set the stage for a RCM reaction. Initial failure of the RCM with a PMB-ether adjacent to the olefins and the difficulty in cleaving the PMB-ether prompted us to change the choice of protecting groups, which then paved the way to the macrocyclic core of palmerolide A.
42. "A Flexible and Unified Strategy for Syntheses of Cladospolides A, B, C and iso-Cladospolide B." Debjani Si, Narayana M. Sekar and Krishna P. Kaliappan, Org. Biomol. Chem. 2011, 9, 6988-6997. DOI:
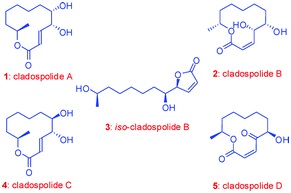
A simple, efficient and flexible strategy for the syntheses of cladospolides A-C and iso-cladospolide B is reported here. This strategy involves Julia-Kocienski olefination and Yamaguchi macrolactonization as key steps, starting from either D-ribose or suitable tartaric acid esters. Although our initial efforts towards cladospolide A involving a ring closing metathetic approach were not successful, changing the mode of ring closure and the use of Julia-Kocienski olefination for the construction of the key intermediate solved this issue and paved the way for the completion of total syntheses of this class of natural products.
41. "A Stereoselective Synthesis of Sugar Derived Chiral β-Lactams ."Rama K. Khangarot and Krishna P. Kaliappan, Eur. J. Org. Chem., 2011, 30, 6117-6127. DOI :
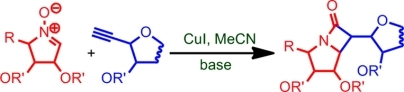
The importance of the β-lactam substructure is exemplified by the several classes of β-lactam antibiotics. Among the synthetic routes available to obtain this interesting scaffold, the Kinugasa reaction is a convergent strategy. In this article, the synthesis of a variety of chiral β-lactams by the Kinugasa reaction between terminal alkynes and nitrones is described. These sugar-derived β-lactams were synthesized by the reaction between cyclic nitrones derived from different sugars, tartrate ester, and alkynes obtained from different sugars. The reaction gave moderate to good yields of β-lactams with high diastereoselectivity, mainly producing a single product. The stereochemical preferences observed in these reactions are also explained.
2010
-
40. "Application of Enyne Metathesis/Diels-Alder Cycloaddition Sequence: A New Versatile Approach to Syntheses of
C-Aryl Glycosides and Spiro C-Aryl Glycosides." A. V. Subrahmanyam, P. Kalanidhi, Krishna P. Kaliappan, Chem. Eur. J. 2010, 16, 8545-8556.DOI :
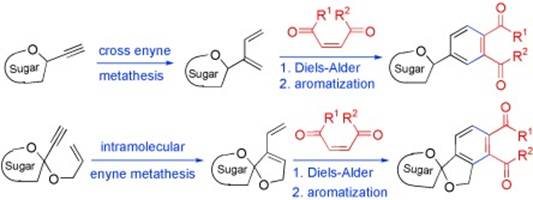
An efficient approach for the synthesis of a variety of C-aryl and spiro-C-aryl glycosides is described. This diversity-oriented strategy employed here relies on a sequential enyne metathesis to generate the 1,3-diene moiety and Diels–Alder reaction with different dienophiles followed by aromatisation. Whereas cross-enyne metathesis with ethylene gas is used to install the 1,3-diene moiety at the anomeric centre for the synthesis of C-aryl glycosides, an intramolecular enyne metathesis on the sugar enyne is performed to generate the 1,3-diene moiety for the synthesis of spiro-C-aryl glycosides. Efforts to extend this strategy to the synthesis of the core structure of natural C-aryl glycoside gilvocarcin are also described. A combination of both C-aryl and spiro-C-aryl glycosides in the same moiety to combine the features thereof has also been accomplished. A tandem enyne metathesis/Diels–Alder reaction/aromatisation has also been attempted to directly access the C-aryl glycosides in one pot albeit in low yield.
39. "A Formal Total Synthesis of Palmerolide A." P. Gowrisankar, S. A. Pujari, Krishna P. Kaliappan Chem. Eur. J. 2010, 16, 5858-5862. DOI :
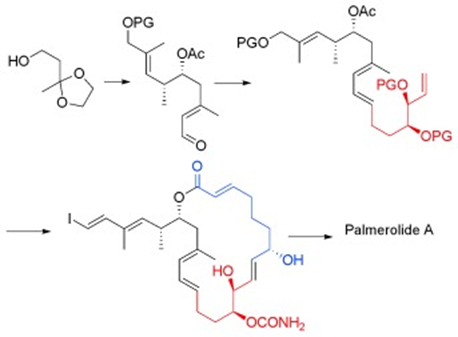
An efficient formal total synthesis of the marine natural product palmerolide A is reported herein, involving 24 longest linear steps. The key features of our synthesis involve a combination of Sharpless epoxidation and Shimizu reaction to construct the syn aldol moiety, a Julia-Kocienski reaction to construct the diene, and ring-closing metathesis to form the macrocycle (see scheme; PG=protecting group).
38. "Synthesis of a Novel Taxa-Oxa-Sugar Hybrid Core Structure by Tandem Cross Enyne Metathesis/IMDA." R. S. Nandurdikar, A. V. Subrhamanyam, Krishna P. Kaliappan, Eur. J. Org. Chem., 2010, 14,2788-2799. DOI :
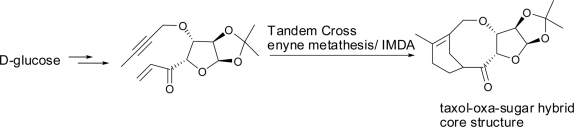
This paper describes our design and efforts in synthesizing new scaffolds with taxol-eleutherobin hybrid core structures and a taxol-sugar hybrid. The synthesis of taxol-eleutherobin hybrids involved the synthesis of the A-ring fragment from carvone and the C-ring fragment from either D-mannose or D-glucose. The Shapiro reaction was used as the key reaction to couple the A- and C-ring fragments of these hybrid structures. Unfortunately, another key reaction (RCM) failed to form the B-ring and essentially the core unit. However, a tandem enyne cross-metathesis/intramolecular Diels-Alder strategy was utilized for the synthesis of a taxa-oxa-sugar hybrid.
37. "Discovery and Syntheses of Superbug Challengers-Platensimycin and Platencin." K. Palanichamy, Krishna P. Kaliappan Chem. Asian J. 2010, 668-703. DOI :
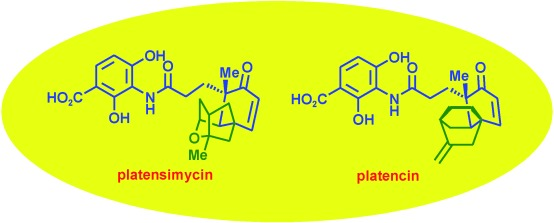
Bacteria have developed resistance to almost all existing antibiotics known today and this has been a major issue over the last few decades. The search for a new class of antibiotics with a new mode of action to fight these multiply-drug-resistant strains, or "superbugs", allowed a team of scientists at Merck to discover two novel antibiotics, platensimycin and platencin using advanced screening strategies, as inhibitors of bacterial fatty acid biosynthesis, which is essential for the survival of bacteria. Though both these antibiotics are structurally related, they work by slightly different mechanisms and target different enzymes conserved in the bacterial fatty acid biosynthesis. This Focus Review summarizes the synthetic and biological aspects of these natural products and their analogues and congeners.
2009
2009
36. "A Versatile Access to Calystegine Analogues as Potential Glycosidases Inhibitors." Krishna P. Kaliappan, P. Das, S. T. Chavan, S. G. Sabharwal J. Org. Chem., 2009, 74, 6266-6274 DOI :
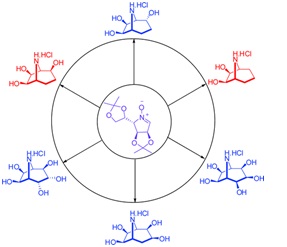
An efficient metathetic strategy and nitrone chemistry have been suitably tethered to construct 8-azabicyclo[3.2.1]octanes as versatile precursors for the synthesis of several calystegine analogues. This synthetic strategy relies on the ability of mannose-derived nitrone to undergo a highly stereoselective nucleophilic addition of various Grignard reagents to access syn orientation of alkenes, which then smoothly undergo ring-closing metathesis (RCM) to provide this framework. These RCM products 18 and 20 have been successfully used as advance precursors to synthesize many calystegine analogues either by syn-dihydroxylation or by hydrogenation and followed by global deprotection. Interestingly, both compounds 36 and 40 exhibited significant noncompetitive inhibition against α-mannosidase and N-acetyl-β-D-gluco-saminidase.
35. "An Expedient Total Synthesis of (-)-Cladospolide A." Krishna P. Kaliappan and D. Si Synlett 2009, 15, 2441-2444. DOI :
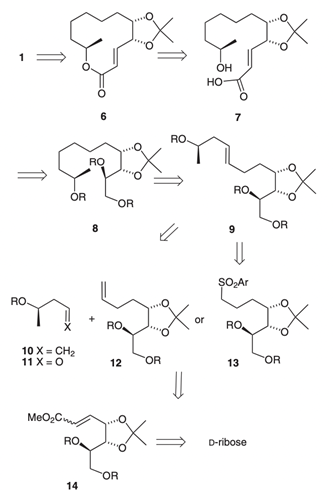
A simple and efficient total synthesis of (-)-cladospolide A is described here, which involves either olefin cross metathesis or Julia-Kocienski olefination and Yamaguchi macrolactonization as key steps.
34. "Click Chemistry on Sugar Derived Alkynes: A Tandem Click-Click Approach to Bistriazoles." Krishna P. Kaliappan, P. Kalanidhi and S. Mahaptra Synlett 2009, 13, 2162-2166. DOI:
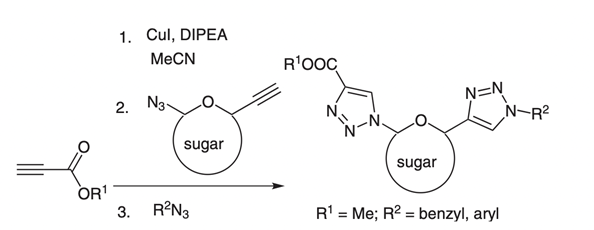
Development of a tandem 'click-click' approach to the formation of successive 1,4-disubstituted 1,2,3-triazole linkages and 'click chemistry' on sugar-derived alkynes are described.
2008
2008
33. "A Rapid Access to New Fluorinated 1,3-Dienes and Benzylic Fluorides via Metathesis on Propargylic Fluorides." Sandip A. Pujari, Krishna P. Kaliappan, Allan Valleix, Danielle Gree and Rene Gree Synlett 2008, 16, 2503-2507. DOI:
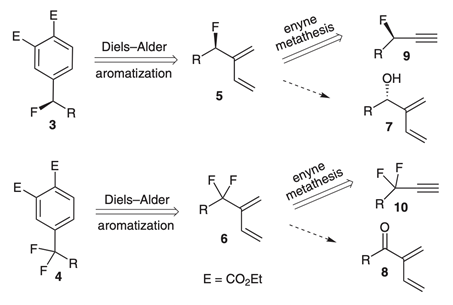
The cross enyne metathesis reaction of propargylic fluoride (+)-12 with ethylene affords the enantioenriched 1,3-diene (+)-14 having fluorine-containing side chain at 2-position in good yield. Upon Diels-Alder reaction, followed by aromatization, this diene affords the new benzylic fluorides (+)-16 and (+)-17 in high ee values. This new strategy has been successfully extended to the corresponding gem-difluoro diene 21 and benzylic fluorides 23 and 24.
32. "Cope-House Cyclization Strategy for the Synthesis of Pyrrolizidines: An Expedient Route to 5-epi-Hyacynthacine A3 and A5." Krishna P. Kaliappan and P. Das Synlett 2008, 6, 841-844. DOI:
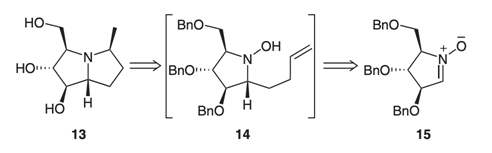
An expedient Cope-House cyclization strategy is reported here for the synthesis of several polyhydroxy pyrrolizidine alkaloids starting from sugar-derived nitrones.
31. "Synthetic Studies on Taxanes: A Domino Enyne Metathesis/Diels-Alder Approach to the AB ring." Krishna P. Kaliappan Velayutham Ravikumar and Sandip A. Pujari J. Chem. Sci. 2008, 120, 205-216. DOI:
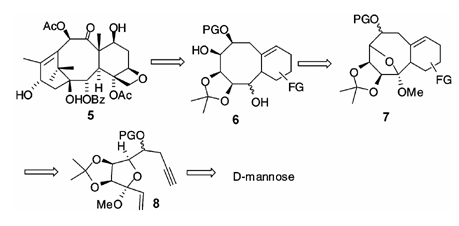
A domino enyne cross-metathesis/intramolecular Diels-Alder reaction has been successfully used to synthesize a bicyclo[5.3.1]undecene, corresponding to AB-ring of taxol without the gem di-methyl group.
2007
2007
30. "Angucylinone Antibiotics: Total Syntheses of YM -181741, (+)-Ochromycinone, (+)-Rubiginone B2, (-)-Tetrangomycin and MM-47755." Krishna P. Kaliappan and V. Ravikumar J. Org. Chem., 2007, 6116-6126. DOI :
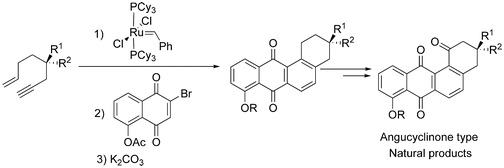
A concise and highly enantioselective route has been developed for the synthesis of angucyclinone-typenatural products. Utilizing this strategy, total syntheses of five natural products YM-181741, (+)-ochromycinone, (+)-rubiginone B2,(-)-tetrangomycin, and MM-47755 have been accomplished in 22%,23%, 19%, 18%, and 12% overall yields, respectively. Our approach for the synthesis of these naturalproducts having the benz[a]anthraquinone skeleton is based on a sequential intramolecular enynemetathesis, intermolecular Diels-Alder reaction (DAR), and aromatization. The intramolecular enynemetathesis reaction was employed for the synthesis of enantiopure 1,3-dienes in excellent yields.Furthermore, the synthesis of YM-181741 as well as structurally similar angucyclinones such as (+)-ochromycinone and (+)-rubiginone B2was achieved via asymmetric enolate alkylation of an oxazolidinonein excellentde. The related angucyclinones (-)-tetrangomycin and MM-47755, bearing a labile tertiaryalcohol, were synthesized via Sharpless asymmetric epoxidation of a known allylic alcohol followed byopening the epoxide with Red-Al. The introduction of oxygen functionality at C-1 in all these naturalproducts was accomplished by photooxygenation under a positive pressure of oxygen.
29. "An Expedient Enantioselective Strategy for the Oxatetracyclic Core of Platensimycin." Krishna P. Kaliappan and V. Ravikumar Org. Lett., 2007, 9, 2417-2430. DOI :
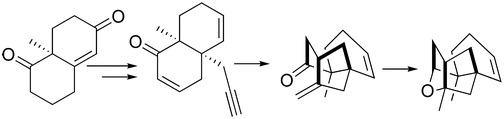
An enantioselective route for the synthesis of oxatetracyclic core of platensimycin is reported for the first time using a 5-exo-trig cyclizationfollowed by intramolecular etherification as key reactions. The requisite dienynone for the radical cyclization is synthesized in eight stepsfrom the Wieland-Miescher ketone employing a Claisen rearrangement.
28. "Synthetic Studies on a Marine Natural Product Palmerolide A: Synthesis of C1-9 and C15-21 fragments." Krishna P Kaliappan and P. Gowrisankar, Synlett 2007, 10, 1537-1540. DOI:
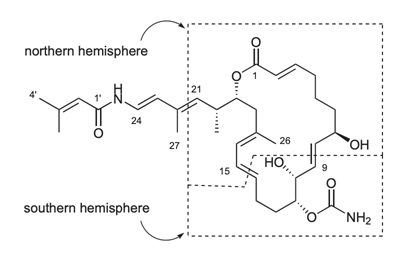
An efficient cross metathesis and Pd-catalyzed allylic re-arrangement have been successfully used to construct the northern hemisphere of a cytotoxic marine natural product, palmerolide A.
27. "First Enantioselective Total Synthesis of the Angucyclinone-Type Anitbiotic YM-181741." Krishna P. Kaliappan and V. Ravikumar Synlett 2007,6, 977-980. DOI:
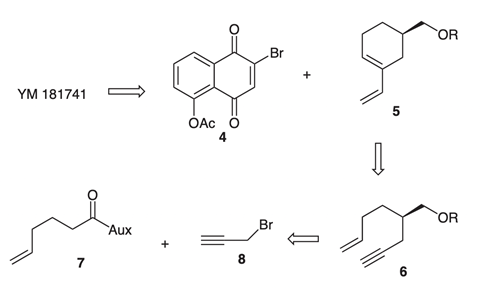
A simple and efficient strategy for angucyclinone anti-biotics is described with the disclosure of first total synthesis of YM-181741.
26. "A New Versatile Strategy for C-Aryl Glycosides." Krishna P. Kaliappan and A. V. Subrahmanyam Org. Lett., 2007, 9, 1121-1124.DOI :
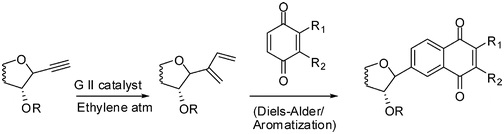
A versatile strategy involving a sequential intermolecular enyne metathesis of C-alkynyl glycosides with ethylene, Diels-Alder, and aromatization reactions is successfully developed to provide a range of C-aryl glycosides.
2006
2006
25. "A Tandem Enyne-Ring Closing Metathesis Approach to the Synthesis of Novel Angularly Fused Dioxatriquinanes." Krishna P. Kaliappan R. S. Nandurdikar and M. M. Shaikh Tetrahedron 2006, 62, 5064-5073. DOI:
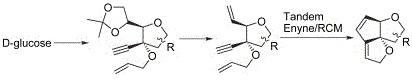
Triquinanes and their oxygenated congeners, oxa- and dioxa-triquinanes, exhibit versatile biological activities in conjunction with synthetically challenging molecular architecture. Owing to these properties, several new strategies have been developed to accomplish the synthesis of these sesquiterpenes. Among the new strategies, cascade radical cyclization strategy has been broadly explored and well studied. Herein, we report our efforts in detail for the synthesis of dioxa-triquinanes using a domino enyne/RCM strategy as the key step. Carbohydrate based synthesis not only allows the use of inexpensive and optically pure starting materials, but also the furanose derivatives, which already possess one of the requisite dihydro-furan moieties in the desired dioxa-triquinane. The other two five-membered rings were constructed simultaneously by the cascade enyne/RCM reaction using the Grubbs' second-generation catalyst. During the course of our synthesis it was observed that the acetonide protection hinders the RCM reaction, after the initial enyne metathesis reaction. The reaction underwent smoothly under argon atmosphere, whilst use of ethylene atmosphere was found to hinder the formation of the tandem enyne/RCM product. The effect of substitution on the key reaction is described here.
24. "Synthesis of a Bicyclo[5.3.1]undecene by a Facile Domino Enyne Cross Metathesis/IMDA." Krishna P. Kaliappan, V. Ravikumar and S. A. Pujari Tetrahedron Lett., 2006, 47, 981-984. DOI :
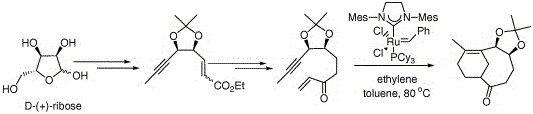
An efficient domino cross-enyne metathesis/intramolecular Diels-Alder reaction is demonstrated for the construction of a bicyclo[5.3.1]undecene.
2005
2005
23. "A Facile Domino Metathetic Route to Thapsigargin Skeleton." Krishna P. Kaliappan and R. S. Nandurdikar, Org. Biomol. Chem. 2005, 3, 3613-3614.DOI:
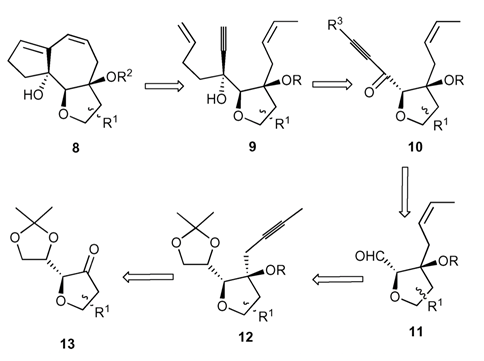
A facile synthesis of a 5,7,5-fused ring system that is presentin thapsigargins belonging to a novel family of sesquiterpene lactones, guainanolides, using domino enyne-RCM isreported here.
22. "Recent Advances in Cascade Enyne/RCM in Organic Synthesis." Krishna P. Kaliappan, Letters in Organic Chemistry, 2005, 2, 678-686. DOI :
A concise highlight on recent advances in cascade/enyne RCM reactions of a range of substrates is discussed. Some of the reported strategies led to total synthesis of biologically active natural products.
21. "Efficient Metathesis Route to the B-ring of Eleutherobin and Other Medium-Sized Cyclic Ethers." Krishna P. Kaliappan and N. Kumar, Tetrahedron 2005, 61, 7461-7469. DOI :
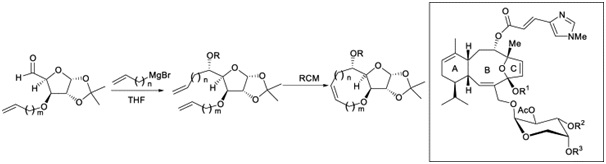
A short and efficient RCM route is reported for the synthesis of the B-ring of eleutherobin and other medium-sized cyclic ethers from readily available 1,2,5,6-diisopropylidene-D-glucose. This strategy is successfully extended to the synthesis of a few bicyclic ethers, which may find applications in the synthesis of novel bicyclic nucleosides.
20. "Design and Synthesis of Novel Oxa-bridged Isoxazolidines and 1,3-Aminoalcohols." Krishna P. Kaliappan, P. Das and N. Kumar, Tetrahedron Lett. 2005, 46, 3037-3040.DOI:
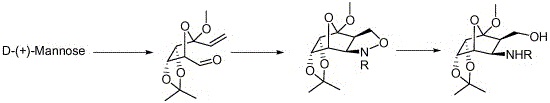
An expedient intramolecular olefin-nitrone cycloaddition (INC) route is reported for the synthesis of a series of noveloxa-bridged isoxazolidines and 1,3-aminoalcohols starting fromD-(+)-mannose-derived nitrones.
19. "Design and Synthesis of Novel Sugar-Oxasteroid-Quinone Hybrids." Krishna P. Kaliappan and V. Ravikumar, Org. Biomol.Chem. 2005, 3, 848-851.DOI:
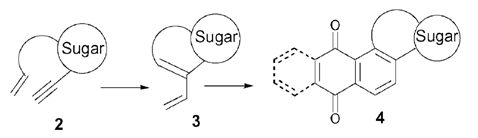
A new class of sugar-oxasteroid-quinone hybrid molecules has been designed and synthesized involving an efficient enyne metathesis/Diels-Alder reaction strategy.
2004
2004
18. "A Cascade Enyne/RCM Approach to Angularly Fused Dioxatriquinanes." Krishna P. Kaliappan and R. S. Nandurdikar, Chem.Commun. 2004, 2506-2507.DOI:
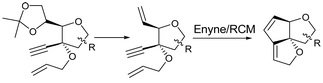
An expedient and first tandem enyne/ring closing metathesis approach on a sugar furanose template leading to a novel angularly fused dioxa-triquinane is described here.
17. "An Expedient Enyne Metathesis Approach to Dysidiolide." Krishna P. Kaliappan and P. Gowrisankar, Tetrahedron Lett.2004, 45, 8207-8209.DOI :
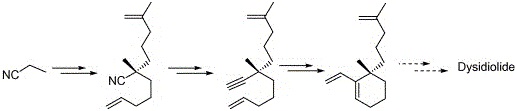
A short and efficient enyne metathesis route is reported for the construction of a key intermediate required in thesynthesis of dysidiolide thus completing its formal synthesis.
2003
2003
16. "A Ring Closing Metathesis Approach to a Synthesis of the B-ring of Eleutherobin." Krishna P. Kaliappan and N. Kumar, Tetrahedron Lett. 2003, 44, 379-381.DOI:
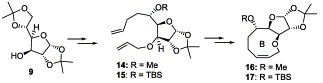
A short and efficient RCM route is reported for the construction of the key nine-membered B ring of eleutherobinstarting from the readily available 1,2,5,6-diisopropylidene-D-glucose.
[ From Duke University, USA ]
-
15. "Combinatorial Discovery of Two-Photon Photoremovable Protecting Groups." Michael C. Pirrung, Wolfgang H. Pieper,
Krishna P. Kaliappan and M. R. Dhananjeyan, Proc. Natl. Acad. Sci. USA ,
2003, 100, 12553-12558.
DOI :
A design principle for a two-photon photochemically removableprotecting group based on sequential one-photon processes hasbeen established. The expected performance of such groups inspatially directed photoactivationphotodeprotection has beenshown by a kinetic analysis. One particular molecular class fittinginto this design, the nitrobenzyl ethers ofo-hydroxycinnamates,has been presented. An initial demonstration of two-photondeprotection of one such group prompted further optimizationwith respect to photochemical deprotection rate. This was accomplished by the preparation and screening of a 135-member indexedcombinatorial library. Optimum performance for>350 nm deprotection in organic solvent was found with 4,5-dialkoxy and -cyanosubstitution in the nitrobenzyl group and 4-methoxy substitutionin the cinnamate.
14. "Dipolar Cycloaddition of Rhodium generated Carbonyl ylides with p-Quinones". M. C. Pirrung and Krishna P. Kaliappan, Org. Lett. 2000, 2, 353-355. DOI :
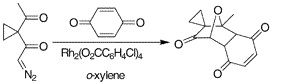
The dipolar cycloaddition of carbonyl ylides generated by the rhodium-catalyzed decomposition of δ- and ε-carbonyl-α-diazoketones with p-quinones leads to both C=O and C=C addition products. The product ratio is solvent- and catalyst-dependent and has been optimized to favor formation of either product. The C=C addition products of naphthoquinones are used in the assembly of structures hybridizing the illudin and anthraquinone anticancer agents.
13. "Pirrung, M. C.; Krishna P. Kaliappan. Dipolar cycloaddition of rhodium-generated carbonyl ylides with p-quinones." Book of Abstracts, 219th ACS National Meeting, San Francisco, CA, March 26-30, 2000 (2000), ORGN-835.
[ From University of Geneva, Switzerland ]
-
12. "Synthesis of [6,n] cis-fused ring compounds via Cr-mediated dearomatization-ring-closing metathesis." E. P. Kundig, A.
Bellido, Krishna P. Kaliappan, A. R. Pape and S Radix, Org. Biomol. Chem.
2006, 4, 342-351.DOI:
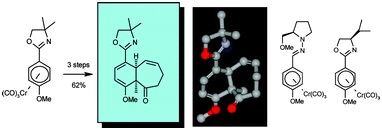
cis-Fused [6,8], [6,7], [6,6] and [6,5] ring systems containing a cyclohexadiene ring unit, a cycloenone ring and a quaternary carbon at the ring junction were obtained in only two steps from [Cr(CO)3(η6-p-methoxyphenyl oxazoline)]. The sequence proceeds via diastereoselective addition of three C-substituents across an arene double bond, followed by allylation and ring closing metathesis (RCM). RAMP-hydrazone and (R)-isopropyloxazoline were used as chiral auxiliaries to provide, after removal of the auxiliaries, the enantiomerically highly enriched [6,7] cis-fused system.
11. "Efficient Access to Fused Ring Compounds via Dearomatization/Ring-Closing Metathesis." E. P. Kundig, A. Bellido, Krishna P. Kaliappan, A. R. Pape and S Radix, Synlett, 2003, 15, 2407-2409. DOI :
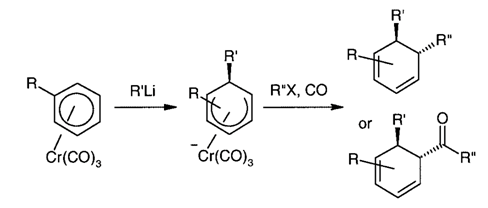
The complex (p-methoxyphenyl oxazoline)Cr(CO)3 is converted in two steps into a cis-fused [6,8] ring system containing a cycohexadiene ring unit, a cyclooctenone ring and a quaternary carbon at the ring junction. The key steps involve a diastereoselective addition of three C-substituents across an arene double bond, followed by an allylation and ring closing metathesis step. cis-Fused [6,7], [6,6], and [6,5] ring systems are also accessible via this methodology.
10. "Transition metal-mediated Dearomatization reactions." A. R. Pape, Krishna P. Kaliappan and E. P. Kundig, Chem. Rev., 2000, 100, 8, 2917-2940. DOI :
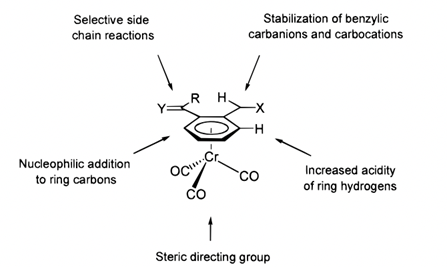
This review describes a wealth of literature covering transition-metal-mediated dearomatization chemistry. The use of chromium, manganese, and osmium has been shown to provide rapid access to compounds which otherwise require long and tedious manipulations. Efficient use of the described methodology can lead to the synthesis of complex organic products with high regio-, chemo-, stereo-, and often enantioselectivity. The chemistry covering intermolecular manipulations is extensive, and there is a broad understanding of the respective limitations for application of each approach. Research within the asymmetric arena is still at the development stage, although numerous methods which deliver asymmetric induction have been designed and adopted. There is no doubt that development in this area will be pursued vigorously and that, with efficient methodology established, applications in the synthesis of organic compounds of high complexity will follow.
9. "Planar Arene Cr(CO)3 in Organic Synthesis". Krishna P. Kaliappan, E. P . Kundig, H. Ratni and D. M. Sigano. Chimia, 1998, 52, 482.
[ From Indian Institute of Science, Bangalore ]
-
8. "Synthetic Studies Towards the Nortriterpene Pfaffic Acid: Synthesis of DEF Fragment via Tandem Radical Cyclization
Rearrangement Reactions." P. J. Biju, Krishna P. Kaliappan and G. S. R. Subba Rao, Arkivoc,
2004, viii, 37-45.DOI :
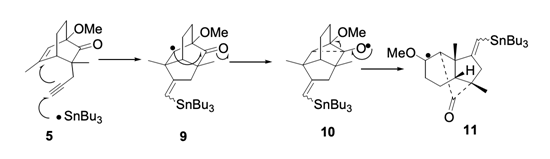
A tandem 5-exo-trig-vinyl-3-exo-radical cyclization-rearrangement reaction and its allylic radical version was developed (8 and 15 to 9 and 16 respectively) for the synthesis of DEF ring system of the nor-tritepene Pfaffic acid 1.
7. "Synthesis Based on Cyclohexadienes: Part 34. A Tandem Cationic Rearrangement-Ene Cyclisation Route to 2-Pupukeanone. P. J. Biju, Krishna P. Kaliappan, Laxmisha, M. S. and G. S. R. Subba Rao, J. Chem. Soc. Perkin Trans 1. 2000, 3714-3718.DOI :
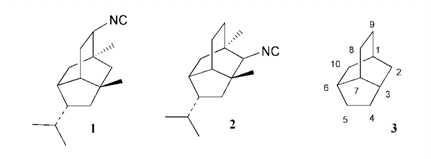
A new strategy for the construction of the isotwistane skeleton is reported from easily available cyclohexadienes, which involves a one-pot cationic skeletal rearrangement and ene cyclisation of a bicyclo[2.2.2]octenone derivative and a cationic rearrangement of a tricyclo[5.3.0.04,8]decane to a [4.3.1.03,7]decane skeleton as the key steps in the synthesis of 2-pupukeanone.
6. "Synthesis Based on Cyclohexadienes: Part 24. A New Total Synthesis of 2-Pupukeanone and a Facile Entry to Copa and Ylanga Sesquiterpene Skeleton". Krishna P. Kaliappan and G. S. R. Subba Rao., J. Chem. Soc. Perkin Trans 1. 1997, 3393-3399. DOI :
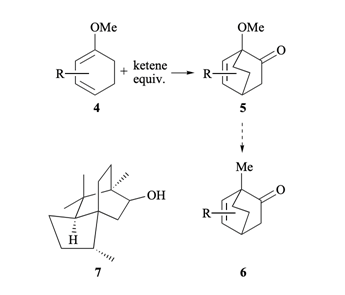
A novel tandem 5-exo-trigallyl and 3-exo-trigradical cyclization and rearrangement to copa and ylangatype sesquiterpene skeletons from easily prepared cyclohexadienes is reported. A new total synthesis ofpupukean-2-one, which belongs to a novel class of sesquiterpenes, involving a 5-exo-trigallyl radicalcyclisation as the key step is also reported.
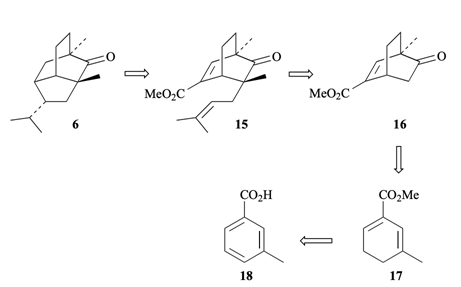
5. "Synthesis Based on Cyclohexadienes: Part 23. Total Synthesis of 5-epi-2-Pupukeanone." Krishna P. Kaliappan and G. S. R. Subba Rao, J. Chem. Soc. Perkin. Trans. 1. 1997, 3387-3392. DOI:
A new strategy for the construction of the isotwistane skeleton is reported from easily availablecyclohexadienes which involves stereoselective alkylation of a bicyclooctenone derivativeand adecarboxylative 5-exo-trigradical cyclisation as the key steps in the total synthesis of 5-epi-pupukean-2-one.
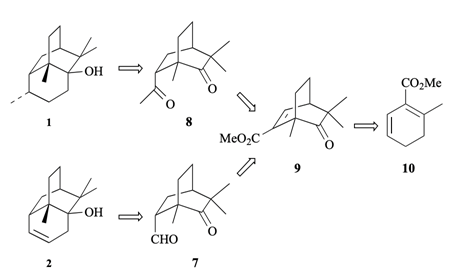
4. "Synthesis Based on Cyclohexadienes: Part 22. Formal Syntheses of Norpatchoulenol and Patchouli Alcohol." Krishna P. Kaliappan and G. S. R. Subba Rao, J. Chem. Soc. Perkin. Trans. 1., 1997, 1385-1389. DOI :
The preparation of 6-endo-formyl-1,3,3-trimethylbicyclo[2.2.2]octan-2-one and 6-endo -acetyl-1,3,3-trimethylbicyclo[2.2.2]octan-2-one, the two key intermediates for the synthesis of patchouli alcohol and norpatchoulenol, is reported by a simple and short method from 2-methylbenzoic acid.
3. "A New Total Synthesis of 2-Pupukeanone." Krishna. P Kaliappan and G. S. R. Subba Rao, Tetrahedron Lett. 1997, 2185-2186.DOI :
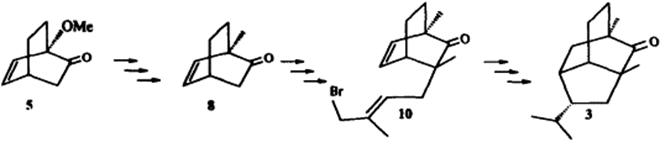
An efficient total synthesis of 2-pupukeanone is reported which involves a 5-exo-trig allyl radical cyclization as the key step to construct the isotwistane skeleton.
2. "An expedient route to the preparation of the key intermediates for the total synthesis of Aphidicolin, Stemodin and Oryzalexin S." Krishna P. Kaliappan and G. S. R. Subba Rao, Tetrahedron Lett. 1996, 8429-8430. DOI :
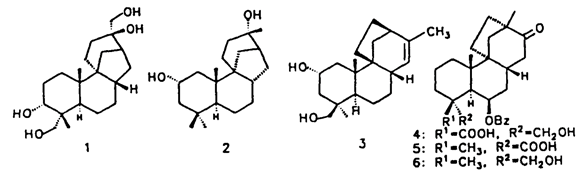
A novel strategy for the construction of tricyclo[6.3.1.01,6]dodecane and tricyclo[7.2.1.01,6]dodecane carbon skeleton present in several complex diterpenes is described.
1. "Tandem 5-exo-trig allyl and 3-exo-trig cyclization and its rearrangement to Copa and Ylanga sesquiterpene skeleton." Krishna P. Kaliappan and G. S. R. Subba Rao, Chem. Commun. 1996, 2331-2332. DOI :
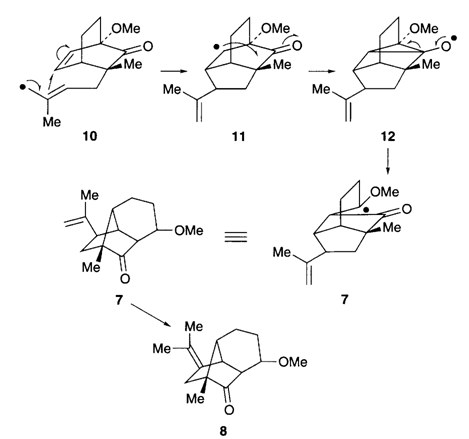
A novel tandem 5-exo-trig allyl and 3-exo-trig radical cyclisation and rearrangement to copa and ylanga type sesquiterpene skeleton is reported.