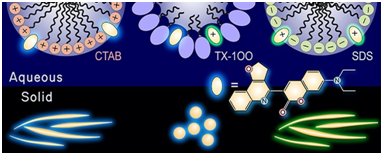
84.
"Surfactant-induced fluorescence enhancement of a quinoline-coumarin derivative in aqueous solutions and dropcast films".
F. Ali, P. E. Hande, D. K. Sahoo, R. Roy,
S. J. Gharpure, A. Datta,
Journal of Photochemistry & Photobiology, A: Chemistry,
2023,
434, 114209.
DOI .
.
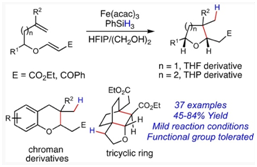
83.
"Iron-Catalyzed Reductive Cyclization of Alkenyl Vinylogous Carbonates for Stereoselective Synthesis of Substituted Tetrahydrofurans, Tetrahydropyrans, and Chromans".
S. J. Gharpure, R. S. Chavan, A. V. Ardhapure,
Adv. Synth. Catal.,
2022,
364, 1-6.
DOI .
.
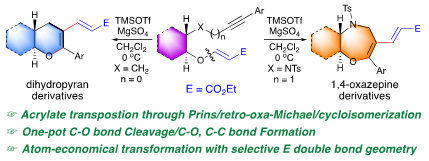
82.
"Transposition of Acrylate Moiety in TMSOTf-Mediated Reaction of Alkynyl Vinylogous Carbonates Gives Heterocyclic Dienes".
S. J. Gharpure, D. J. Fartade, K. S. Gupta, R. K. Patel ,
Chem. Commun.,
2022,
58, 9762-9765.
DOI .
.
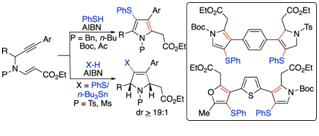
81.
"Protecting Group-Dependent Synthesis of Densely Substituted Dihydropyrroles v/s Pyrroles via 5-Exo-trig Cascade Radical Cyclization to Alkynyl Vinylogous Carbamates".
S. J. Gharpure , S. Kumari,
J. Org. Chem.,
2022,
87, 6781-6793.
DOI .
.
80.
"Total synthesis of myristinins A-F and 3?-hydroxy-5,7-dimethoxy-4-O-2?-cycloflavan by iterative generation of o-quinone methides".
S. J. Gharpure ,S. Jegadeesan, D. S. Vishwakarma,
New J. Chem,
2022,
46, 5425-5437.
DOI .
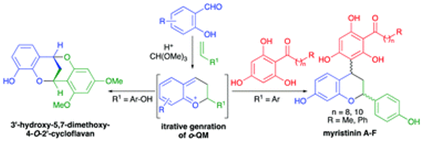
.
79.
"Room temperature HFIP/Ag-promoted palladium-catalyzed C-H functionalization of benzothiazole with iodoarenes".
S. Kori, Y. Bhujbal, K. Vadagaonkar, A. R. Kapdi, S. P. Kommyreddy,
S. J. Gharpure ,
Chem. Commun.,
2022,
DOI .

.
78.
"TMSOTf-Mediated Formal [4 + 2] Cycloaddition-Retro-aza-Michael Cascade of Vinylogous Carbamates for the Synthesis of Highly Fluorescent Pyridocarbazoles".
S. J. Gharpure, P. E. Hande, S. K. Pandey, G. Samala, ,
J. Org. Chem.,
2021,86, 16652-16665.
DOI .
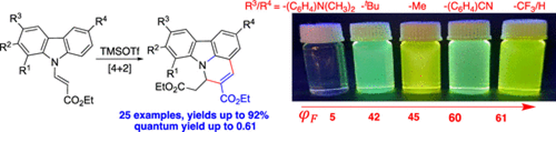
.
77.
"Acid-catalysed iterative generation of o-quinone methides for the synthesis of dioxabicyclo[3.3.1]nonanes: total synthesis of myristicyclins A-B".
S. J. Gharpure, S. Jegadeesan, D. S. Vishwakarma, ,
Chem. Commun.,
2021,57, 13333-13336.
DOI .
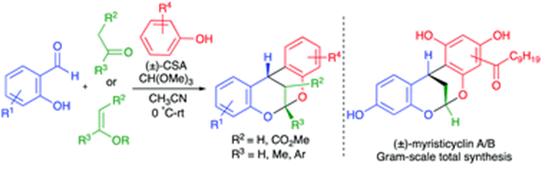
.
76.
"Recent Advances in Small Molecule-Based Intracellular pH Probes".
P. E. Hande, Y. G. Shelke, A. Datta,
S. J. Gharpure ,
ChemBioChem.,
2021,
DOI .
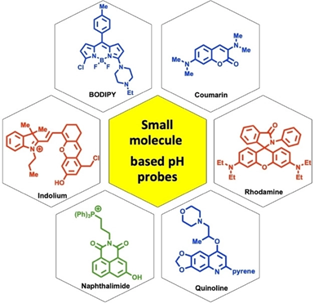
.
75.
"Recent advances in the synthesis of pyrrolo[1,2-a]indoles and their derivatives".
Y. G. Shelke, P. E. Hande,
S. J. Gharpure,
Org. Biomol. Chem.,
2021,19, 7544-7574.
DOI .
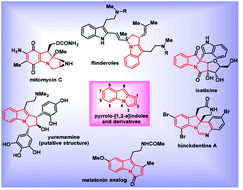
.
74.
"Formal [4+2] Cycloaddition of o-Aza-Quinone Methide for the Synthesis of 1,4-Heterocycle-Fused Quinolines".
S. J. Gharpure, S. K. Nanda, D. J. Fartade,
Adv. Synth. Catal.,
2021,363, 2562.
DOI .
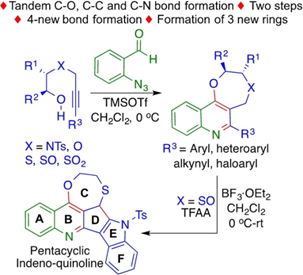
.
73.
"Regioselective Synthesis of Halotriazoles and their Utility in Metal Catalyzed Coupling Reactions".
S. J. Gharpure, S. Naveen, R. S. Chavan, Padmaja ,
Eur. J. Org. Chem.,
2020,6870.
DOI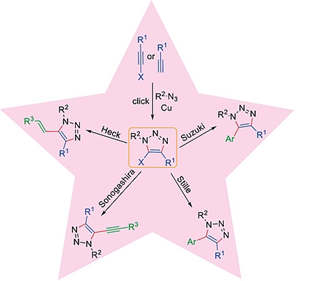
.
72.
"Design and Expeditious Synthesis of Quinoline-Pyrene-Based Ratiometric Fluorescent Probes for Targeting Lysosomal pH".
P. E. Hande, M. Mishra, F. Ali, S. Kapoor, A. Datta,
S. J. Gharpure ,
ChemBioChem.,
2020,21, 1492-1498.
DOI .
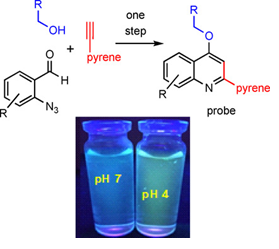
.
71.
"Domino Hydroalkoxylation-[4+2]-Cycloaddition for Stereoselective Synthesis of 1,4-Heterocycle-Fused Chromenes: Rapid Access to the [6-6-7-6] Tetracyclic Core of Cytorhizhins B-D".
S. J. Gharpure, S. K. Nanda, D. J. Fartade, D. S. Vishwakarma ,
Eur. J. Org. Chem.,
2020,6892.
DOI 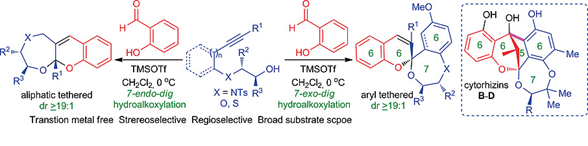
.
70.
"Lewis Acid Catalyzed Intramolecular [4+2] Cycloaddition of In Situ Generated Aza-Quinone Methides for the Stereoselective Synthesis of Furo/pyrano[3,2-c]tetrahydroquinolines".
S. J. Gharpure, D. S. Vishwakarma ,
Eur. J. Org. Chem.,
2020,6887
DOI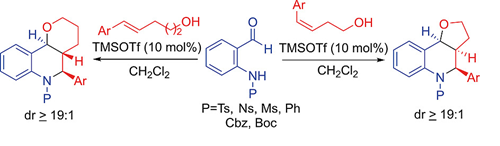
.
69.
"Notorious Radicals and Their Fate".
Padmaja,
S. J. Gharpure ,
INTERWOVEN Interdiscip. J. Navrachana Univ.,
2020,2(2), 1. (Invited article).
68.
"Expeditious Diastereoselective Synthesis of Medium Ring Heterocycle-Fused Chromenes via Tandem 8/9-endo-dig and 8-exo-dig Hydroalkoxylation-Formal-[4+2]-Cycloaddition".
S. J. Gharpure, S. K. Nanda, D. J. Fartade,
Org. Biomol. Chem.,
2019,
DOI .
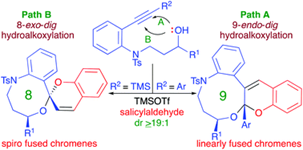
.
67.
"TMSOTf Mediated '5/6-endo-dig' Reductive Hydroamination for the Stereoselective Synthesis of Pyrrolidine and Piperidine derivatives".
S. J. Gharpure, D. S. Vishwakarma, R. K. Patel,
Chem. Commun.,
2019, 55, 6858-6861.
DOI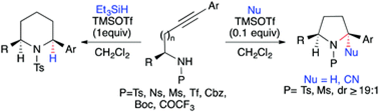
.
66.
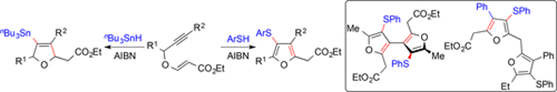
"Cascade Radical Cyclization on Alkynyl Vinylogous Carbonates for the Divergent Synthesis of Tetrasubstituted Furans and Dihydrofurans".
S. J. Gharpure, Padmaja, V. Prasath, Y. G. Shelke,
Org. Lett. 2019, 21, 223.
DOI .
.
65.
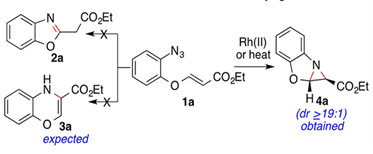
"Transition-Metal Acetate-Promoted Intramolecular Nitrene Insertion to Vinylogous Carbonates for Divergent Synthesis of Azirinobenzoxazoles and Benzoxazines".
S. J. Gharpure, S. Naveen, G. Samala, D. S. Vishwakarma,
Chem. Eur. J. 2019, 25, 1456.
DOI .
.
64.
"Homogeneous Catalysis: Powerful Technology for the Modification of Important Bio Molecules".
Y. G. Shelke, A. Yashmeen, A. V. A. Gholap,
S. J. Gharpure, A. R. Kapdi,
Chem. Asian J.,
2018, 13, 2991.
DOI,
(Invited review) .
.
63.
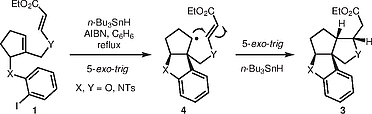
"Cascade Radical Cyclization to Vinylogous Carbonates/Carbamates for the Synthesis of Oxa- and Aza-Angular Triquinanes: Diastereoselectivity Depends on the Ring Size of Radical Precursor".
S. J. Gharpure, P. Niranjana, S. K. Porwal,
Synthesis,
2018, 50, 2954.
DOI, (Invited article, Special Topic on Modern Radical Methods and their Strategic Applications in Synthesis) .
.
62.
"Lewis Acid Mediated "endo-dig" Hydroalkoxylation?Reduction on Internal Alkynols for the Stereoselective Synthesis of Cyclic Ethers and 1,4-Oxazepanes".
S. J. Gharpure, D. S. Vishwakarma, S. K. Nanda,
Org. Lett. 2017, 19, 6534.
DOI .
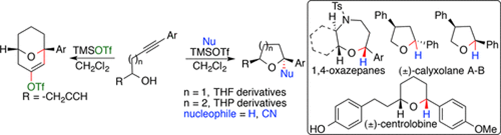
.
61.
"Synthesis of Benzo[1,4]heterocycles using Palladium Catalyzed Heck Reaction to Vinylogous Carbonates/Carbamates: Unexpected Formation of Indoles via Carbopalladation Intercepted by Nucleopalladation".
S, J. Gharpure, D. Anuradha,
Org. Lett. 2017, 19, 6136.
DOI .
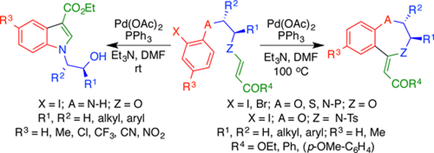
.
60.
"Lewis Acid Mediated Cascade Friedel?Craft/Alkyne Indol-2-yl Cation Cyclization/Vinyl Cation Trapping for the Synthesis of N?Fused Indole Derivatives".
S. J. Gharpure, Y. G. Shelke, Org. Lett. 2017, 19, 5406. DOI.
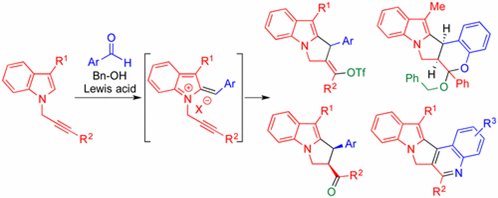 .
.
59. "Cascade Radical Cyclization of N-Propargylindoles: Substituents Dictate Stereoselective Formation of N-Fused Indolines versus Indoles".
S. J. Gharpure, Y. G. Shelke, Org. Lett. 2017, 19, 5022. DOI.
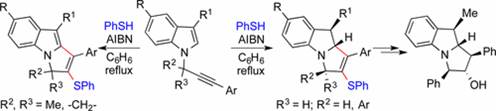 .
.
58. "Enantiospecific Total Synthesis of (+)-3-epi-Epohelmin A Using a Nitrogen-Substituted Donor-Acceptor Cyclopropane".
S. J. Gharpure, L. N. Nanda, D. Kumari, Eur. J. Org. Chem. 2017, 3917. DOI.
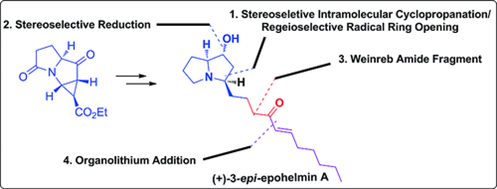 .
.
57. "Metal-free Hydroalkoxylation-Formal [4+2] Cycloaddition Cascade for the Synthesis of Ketals".
S. J. Gharpure, S. K. Nanda, Padmaja, Y. G. Shelke, Chem. Eur. J. 2017, 23, 10007. DOI
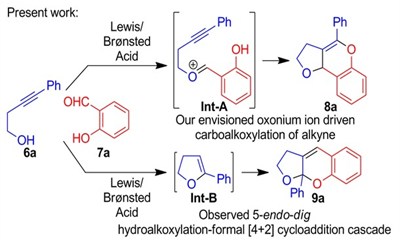 .
.
56. "Application of Oxygen/Nitrogen Substituted Donor-Acceptor Cyclopropanes in the Total Synthesis of Natural Products".
S. J. Gharpure, L. N. Nanda, Tetrahedron Lett. 2017, 58, 711. DOI. (Invited digest article)..
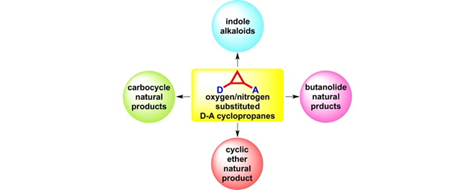 .
.
55. Lewis Acid Promoted Oxonium Ion Driven Carboamination of Alkynes for the Synthesis of 4-Alkoxy Quinolines.
Gharpure, Santosh J.; Nanda, Santosh K.; Adate, Priyanka A.; Shelke, Yogesh G. Journal of Organic Chemistry (2017), 82(4), 2067-2080.
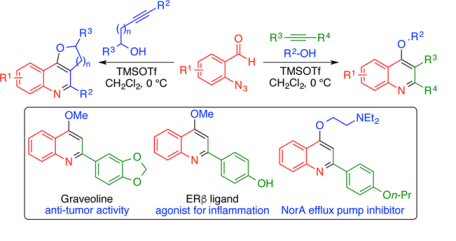 .
.
54. Photophysics of Diphenylbutadiynes in Water, Acetonitrile-Water, and Acetonitrile Solvent Systems: Application to Single Component White Light Emission.
Pati, Avik Kumar; Jana, Rounak; Gharpure, Santosh J.; Mishra, Ashok K. Journal of Physical Chemistry A (2016), 120(29), 5826-5837.
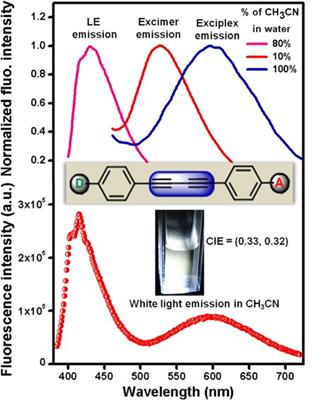 .
.
53. White Light Emission in Butadiyne Bridged Pyrene-Phenyl Hybrid Fluorophore: Understanding the Photophysical Importance of Diyne Spacer and Utilizing the Excited-State Photophysics for Vapor Detection.
Pati, Avik Kumar; Gharpure, Santosh J.; Mishra, Ashok K. Journal of Physical Chemistry A (2016), 120(29), 5838-5847,
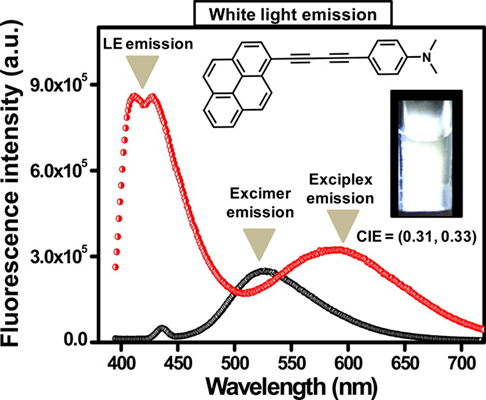 .
.
52. Stereoselective synthesis of thiazino[4,3-a]indoles using the thia-Pictet-Spengler reaction of indoles bearing N-tethered thiols and vinylogous thiocarbonates.
Gharpure, Santosh J.; Nanda, Santosh K. Organic & Biomolecular Chemistry (2016), 14(24), 5586-5590.
51. Stereoselective Synthesis of Donor-Acceptor Cyclopropapyranone by Intramolecular Cyclopropanation of Vinylogous Carbonates: Application to the Total Synthesis of (±)-Diospongin B.
Gharpure, Santosh J.; Mane, Sumit P.; Nanda, Laxmi Narayan; Shukla, Manoj Kumar. Israel Journal of Chemistry (2016), 56(6-7), 553-557.
50. Contrasting Solid-State Fluorescence of Diynes with Small and Large Aryl Substituents: Crystal Packing Dependence and Stimuli-Responsive Fluorescence Switching.
Pati, Avik Kumar; Gharpure, Santosh J.; Mishra, Ashok K. Journal of Physical Chemistry A (2015), 119(42), 10481-10493.
49. Stereoselective synthesis of 2,3-disubstituted indoline, pyrrolidine and cyclic ether-fused 1,2-dihydroquinoline derivatives using alkyne iminium ion cyclization of vinylogous carbamates: switch of regioselectivity using an internal hydroxy group as a nucleophile.
Gharpure, Santosh J.; Prasath, V.; Kumar, Vinod, Chem. Comm., 2015, 13623-13626.
48. Counter Ion Dependent Alkyne Iminium Ion Cyclization for Divergent Synthesis of N-Fused Indolylidine, Indole and Indoline Derivatives Promoted by the Lewis/Bronsted Acid.
S. J. Gharpure, Y. G. Shelke and D. P. Kumar, Org. Lett.(2015), 17(8), 1926-1929 .
47. Stereoselective Synthesis of cis-2,6-DisubstitutedMorpholines and 1,4-Oxathianes by Intramolecular Reductive Etherification of 1,5-Diketones.
S. J.Gharpure, D. Anuradha, J. V. K Prasad, and P. S. Rao, Eur. J. Org. Chem., 2015, 86-90.
46.Donor-Acceptor Substituted Cyclopropane to Butanolide and Butenolide Natural Products: Enantiospecific First Total Synthesis of (+)-Hydroxyancepsenolide.
S. J. Gharpure, Laxmi Narayan Nanda and M. K. Sukla, Org. Lett.2014,16, 6424?6427.
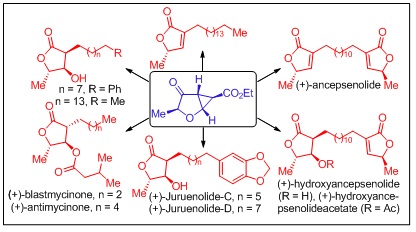
45.On the Photophysics of ButadiyneBridged Pyrene-Phenyl Molecular Conjugates: Multiple Emissive Pathways Through Locally Excited, Intramolecular Charge Transfer and ExcimerStates.
A. K.Pati, S. J. Gharpure and A. K. Mishra,Faraday Discuss.2014.(Invited)
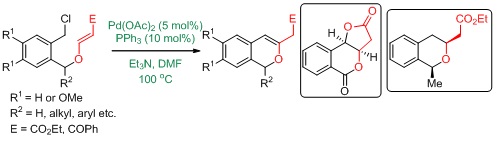
44.Synthesis of IsochromeneDerivatives using an Intramolecular Benzylic C(sp3)-C(sp2) Bond Forming Heck Reaction on Vinylogous Carbonates.
S.J. Gharpure ,Yogesh, G. Shelke and S.R.B. Reddy,RSC Adv.,2014, 4, 46962-46965.
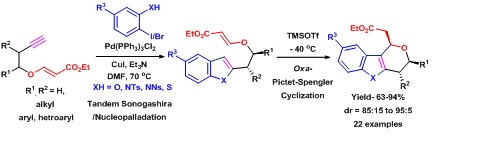
43. Stereoselective Synthesis of C-fused Pyranoindoles, Pyranobenzofurans and PyranobenzothiopheneScaffolds using Oxa-Pictet-Spengler Type Teaction of Vinylogous Carbonates.
S. J. Gharpure and V. Prasath,Org. Biomol. Chem., 2014,12, 7397-7409.
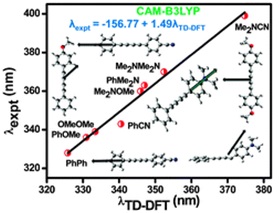
42.Substituted DiphenylButadiynes: a Computational Study of Geometries and Electronic Transitions using DFT/TD-DFT.
A. K.Pati, S. J. Gharpure and A. K. Mishra, Phys. Chem. Chem. Phys.,2014, 16, 14015-14028.
41.Tandem Nucleophilic Addition/Oxa-Michael Reaction for the Synthesis of cis-2,6-DisubstitutedTetrahydropyrans.
S. J. Gharpure, J. V. K. Prasad and KalisankarBera,Eur. J. Org. Chem., 2014,3570-3574.
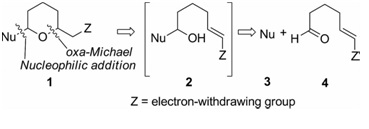
40. Stereoselective Synthesis of Oxa-Bowls by Nucleophilic Addition to Oxonium Ions: Observation of Nucleophile-Dependent Hydride Migration.
S. J. Gharpure and S. K. Porwal, Eur. J. Org. Chem., 2013, 7277-7281.
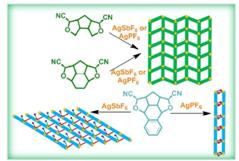
39. Coordination Polymers via Self-assembly of Silver(I) and cis-Bisnitrile-oxa-bowl Derivatives.
P. Niranjana, A. Pati, S. K. Porwal, V. Ramkumar, S. J. Gharpure and D. K. Chand, CrystEngComm.,2013.15, 9623-9633.
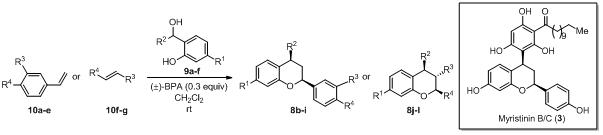
38. Hetero Diels-Alder Reaction of Olefin with o-Quinone Methides Generated Using (±) Binolphosphoric Acid for the Stereoselective Synthesis of 2,4-Diarylbenzopyrans: Application to the Formal Synthesis of Myristinin B/C.
S. J. Gharpure, A. M. Sathiyanarayananand P. K. Vuram, RSC Adv., 2013,3, 18279-18282.
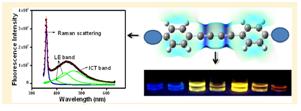
37. Deciphering the Photophysical Role of Conjugated Diyne in Butadiynyl Fluorophores: Synthesis, Photophysical and Theoretical Study.
A. K. Pati, M. Mohapatra, P. Ghosh, S. J. Gharpure and A. K. Mishra, Phys. Chem. A.,2013, 117, 6548-6560.
36. Stereoselective synthesis of benzoxepines using tandem alkylation-Michael addition to vinylogous carbonates.
S. J. Gharpure and S. R. B. Reddy, Eur. J. Org. Chem., 2013, 2031-2038.
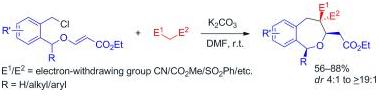
35. Stereoselective Synthesis of Substituted 1,4-Oxazepanes by Intramolecular Reductive Etherification.
S. J. Gharpure and J. V. K. Prasad, Eur. J. Org. Chem., 2013, 2076-2079.
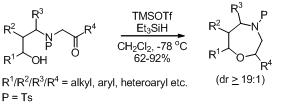
34. Synthesis of Oxa-, Aza- and Thia-Bowls and Cages.
S. J. Gharpure and S. K. Powal, Org. Prep. Proc. Int., 2013, 45, 81. (Invited review).
33. Stereoselective Synthesis of Oxa- and Aza-Angular Triquinanes Using Tandem Radical Cyclization to Vinylogous Carbonates and Carbamates.
S. J. Gharpure, P. Niranjana and S. K. Powal, Org. Lett., 2012, 14, 5476. (Highlighted in Synfacts, 2013, 9(1), 0033).
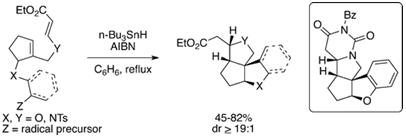
32. Stereoselective Synthesis and Applications of Nitrogen Substituted Donor-Acceptor Cyclopropanes (N-DACs) in the Divergent Synthesis of Azacycles.
S. J. Gharpure, U. Vijayasree and S. R. B. Reddy, Org. Biomol. Chem., 2012, 10, 1735.
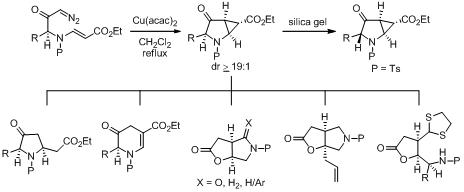
31. Stereoselective Synthesis of C-Substituted Morpholine Derivatives using Reductive Etherification Reaction: Total Synthesis of Chelonin C.
S. J. Gharpure and J. V. K. Prasad, J. Org. Chem., 2011, 76, 10325 .
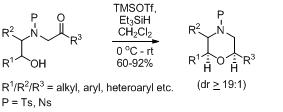
30. Enantioselective Total Synthesis of (+)-Hagen's Gland Lactones.
S. J. Gharpure, L. N. Nanda, and M. K. Shukla, Eur. J. Org. Chem., 2011, 6632. (Among the top 10 most downloaded paper for October 2011).
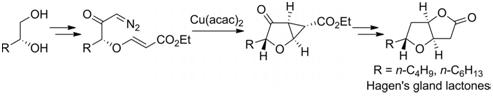
29. Stereoselective Synthesis of 2,3-Disubstituted Dihydrobenzofuran using Alkyne Prins Type Cyclization to Vinylogous Carbonates.
S. J. Gharpure, V. Prasath, J. Chem. Sci., 2011, 123, 943. (invited article - International Year of Chemistry) .
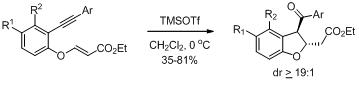
28. Stereoselective synthesis of oxazino[4,3-a]indoles employing oxa-Pictet-Spengler reaction of indoles bearing N-tethered vinylogous carbonate.
S. J. Gharpure, A. M. Sathiyanarayanan, Chem. Commun., 2011, 47, 3625.
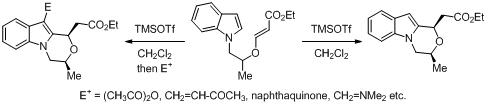
27. Alkyl radical cyclization to vinylogous carbonates for the stereoselective synthesis of unsymmetrical dioxa-cage compounds: Effect of conformation on the rate of cyclization v/s reduction.
S. J. Gharpure, S. K. Porwal, Tetrahedron, 2011, 67, 1216 .
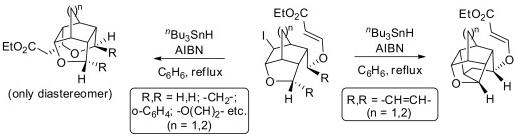
26. Tandem SN2-Michael Addition to Vinylogous Carbonates for the Stereoselective Construction of 2,3,3,5-Tetrasubstituted Tetrahydrofurans.
S. J. Gharpure, S. R. B. Reddy, Tetrahedron Lett., 2010, 51, 6093.
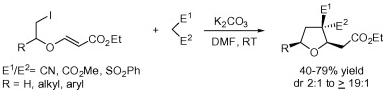
25. Tandem Radical Cyclization Based Strategy for the Synthesis of Oxa- and Aza-Cages: A Case of Fragmentation v/s Cyclization.
S. J. Gharpure, S. K. Porwal, Tetrahedron Lett., 2010, 51, 3324 .
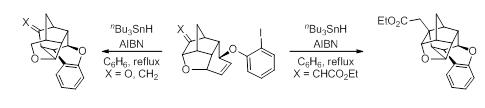
24. Stereoselective Synthesis of Donor-Acceptor Substituted Cyclopropafuranones by Intramolecular Cyclopropanation of Vinylogous Carbonates: Divergent Synthesis of Tetrahydrofuran-3-one, Tetrahydropyran-3-one and Lactones.
S. J. Gharpure, M. K. Shukla, U. Vijayasree Org. Lett., 2009, 11, 5466.
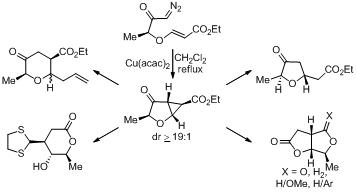
23. Topologically Driven Tandem Radical Cyclization Based Strategy for the Synthesis of Oxa and Aza-Cages.
S. J. Gharpure, S. K. Porwal, Tetrahedron Lett., 2009, 50, 7162 .
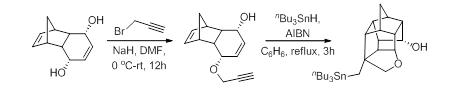
22. Tandem Alkylation-Michael Addition to Vinylogous Carbonates for the Stereoselective Construction of 2,3,3,6-Tetrasubstituted Tetrahydropyrans.
S. J. Gharpure, S. R. B. Reddy, Org. Lett., 2009, 11, 2519.
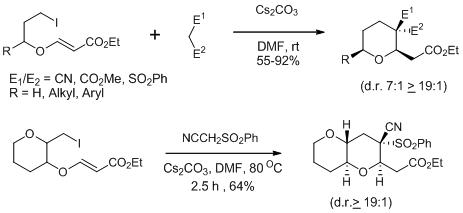
21. o-Quinone Methide Based Approach to Isoflavans: Application to the Total Syntheses of Equol, 3'-Hydroxyequol and Vestitol.
S. J. Gharpure, A. M. Sathiyanarayanan, P. Jonnalagadda, Tetrahedron Lett., 2008, 49, 2974.
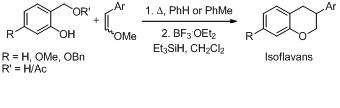
20. Stereoselective Synthesis of New Oxa-Cages via Alkyl Radical Cyclization to Vinylogous Carbonates.
S. J. Gharpure, S. K. Porwal, Synlett, 2008, 242 .
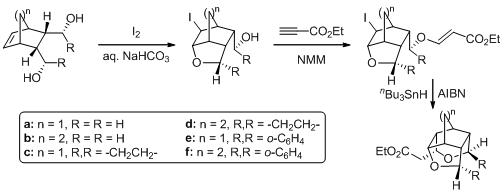
19. Stereoselective Synthesis of 1,2,2-Trisubstituted Indane Derivatives Using Tandem SN2-Michael Addition Sequence.
S. J. Gharpure, S. R. B. Reddy, U. Sanyal, Synlett, 2007, 1889 .
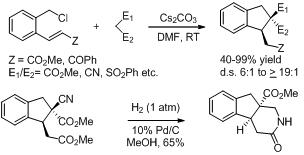
18. An Enantiospecific Strategy to All Four Diastereomers of A-Ring Enyne Synthon of 1 ,25- Dihydroxyvitamin D3.
A. Srikrishna, S. J. Gharpure, P. P. Kumar, Ind. J. Chem. B 2006, 45B, 2736.
17 An Enantiospecific Synthesis of 2-Pupukeanone.
A. Srikrishna, P. R. Kumar, S. J. Gharpure, Ind. J. Chem. B 2006, 45B, 1909.
16. A Central Strategy for Converting Natural Products into Fluorescent Probes.
M. D. Alexander, M. D. Burkart, M. S. Leonard, P. Portonovo, B. Liang, X. Ding, M. M. Joulli, B. M. Gulledge, J. B. Aggen, A. R. Chamberlin, J. Sandler, W. Fenical, J. Cui, S. J. Gharpure, A. Polosukhin, H.-R. Zhang, P. A. Evans, A. D. Richardson, M. K. Harper, C. M. Ireland, B. G. Vong, T. P. Brady, E. A. Theodorakis, J. J. La Clair, Chem. Bio. Chem. 2006, 7, 409.
15. Enantiospecific Synthesis of (+)-2-Thiocyanatoneopupukeanane.
A. Srikrishna, S. J. Gharpure, Proc. of AP Akademi of Sciences 2005, 9, 115.
14. Enantioselective Total Synthesis of the Potent Antitumor Agent (-)-Mucocin using a Temporary Silicon-Tethered (TST) Ring-Closing Metathesis (RCM) Cross-Coupling Reaction.
P. A. Evans, J. Cui, S. J. Gharpure, A. Polosukhin, H. -R. Zhang, J. Am. Chem. Soc. 2003, 125, 14702.
13. Stereoselective Construction of cis-2,6-Disubstituted Tetrahydropyrans via the Reductive Etherification of ?-Trialkylsilyloxy Substituted Ketones: Total Synthesis of (-)-Centrolobine.
P. A. Evans, J. Cui, S. J. Gharpure, Org. Lett. 2003, 5, 3883.
12. Stereoselective Construction of Cyclic Ethers using a Tandem Two-Component Etherification: Elucidation of the Role of Bismuth Tribromide.
P. A. Evans, J. Cui, S. J. Gharpure, R. J. Hinkle, J. Am. Chem. Soc. 2003, 125, 11456.
11. Stereochemistry of the Marine Sesquiterpene 2-Thiocyanatoneopupukeanane: Crystal Structure of Neopupukean-2-yl 4-nitrobenzoate.
A. Srikrishna, S. J. Gharpure, P. Venugopalan, Ind. J. Chem. B 2003, 42B, 129.
10. Chiral Synthons from Carvone. Part 56. Enantiospecific Synthesis of (-)-4-Thiocyanatoneopupukeanane.
A. Srikrishna, S. J. Gharpure, ARKIVOC 2002, 7, 52.
9. A Ring Closing Metathesis Based Approach for the Spiroannulation of Cyclopentanes and Cyclohexanes. Formal Synthesis of (±)-Acorones.
A. Srikrishna, M. S. Rao, S. J. Gharpure, N. C. Babu, Synlett 2001, 1986.
8. Enantiospecific Synthesis of B-seco-C-Aromatic Taxanes.
A. Srikrishna, T. J. Reddy, P. P. Kumar, S. J. Gharpure, Ind. J. Chem. B 2001, 40B, 905.
7. Enantiospecific Total Synthesis of (-)-4-Thiocyanatoneopupukeanane.
A. Srikrishna, S. J. Gharpure, J. Org. Chem. 2001, 66, 4379.
6. An Enantiospecific Synthesis of (-)-2-Pupukeanone via a Rhodium Carbenoid C-H Insertion Reaction.
A. Srikrishna, P. R. Kumar, S. J. Gharpure, Tetrahedron Lett. 2001, 42, 3929.
5. Novel Formation of Chloromethanesulfinates in the Methanesulfonylation Reaction of Hindered Alcohols.
A. Srikrishna, S. J. Gharpure, Synlett 2000, 1354.
4. Enantiospecific Total Synthesis of both Enantiomers of 2-Thiocyanatoneopupukeanane from (R)-Carvone.
A. Srikrishna, S. J. Gharpure, J. Chem. Soc. Perkin Trans. 1 2000, 3191.
3. A Simple, Enantiospecific Approach to both Enantiomers of 1?,25-Dihydroxyvitamin D-3 A-Ring Precursors from (R)-Carvone.
A. Srikrishna, S. J. Gharpure, P. P. Kumar, Tetrahedron Lett. 2000, 41, 3177.
2. Enantiospecific First Total Synthesis of (-)-4-Thiocyanatoneopupukeanane.
A. Srikrishna, S. J. Gharpure, Tetrahedron Lett. 1999, 40, 1035.
1. An Intramolecular Rhodium Carbenoid C-H Insertion Approach to Chiral Isotwistanes. Synthesis of (-)-Neopupukean-4,10-dione and (-)-Neopupukean-10-one.
A. Srikrishna, S. J. Gharpure, Chem. Commun. 1998, 1589.
 .
. .
. .
. .
. .
. .
. .
. .
.
 .
.
 .
.
 .
.
 .
.
 .
.
 .
.
 .
.
 .
.
 .
.
 .
.
 .
.
 .
. .
.
 .
.
 .
.
 .
.
 .
.
 .
.
 .
.
 .
.
 .
.
 .
.
 .
.































