Research :
In the broad area of Organic Chemistry, the main focus of Prof. Gharpure's research has been the development of new synthentic methods, which would be applicable for the synthesis of natural and unnatural products of biological relevance.
Synthetic Methodology :
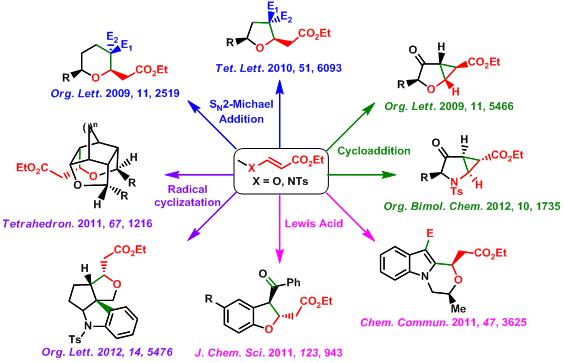
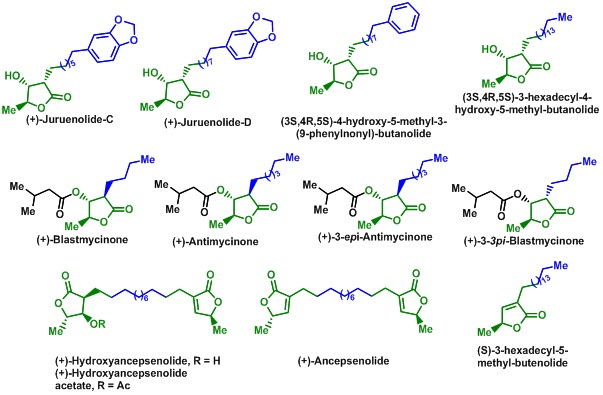
We have used vinylogous functional groups, namely vinylogous carbonate and carbamate, in the stereoselective synthesis of oxa- and aza-cycles. The reactivity of vinylogous carbonates/carbamates was studied under a variety of conditions. Thus, our group has used radical cyclisation to vinylogous carbonates/carbamates for the stereoselective synthesis of new oxa-cages as well as angular oxa- and aza-triquinanes. An efficient strategy for the synthesis of tetrahydrofurans (THFs), tetrahydropyrans (THPs) and oxepane derivatives has been developed employing a tandem SN2-Michael addition to vinylogous carbonates. On the other hand, intramolecular cyclopropanation of vinylogous carbonates/carbamates using carbenes led to the oxygen and nitrogen bearing donor-acceptor substituted cyclopropanes (DACs). These DACs could be converted into diversely functionalized THFs, THPs, lactones, pyrrolidine, piperidine and lactam derivatives, which is again explored for the total synthesis of bioactive molecules such as butanolide and butenolide based natural products.Vinylogous carbonates/carbamates were also found to give useful reactions in the presence of Lewis acids undergoing intramolecular Pictet-Spengler as well as Prins type cyclizations leading to N-fused oxazinoindoles, dihydrobenzofurans and dihydroindole derivatives.
Natural Product Synthesis :
Apart from using vinylogous functional groups in the synthesis of oxa- and aza-cycles, our group has developed efficient strategies for the synthesis of flavans and isoflavans. Flavans and isoflavans are class of compounds, which are thought to be responsible for biological activity of traditional Chinese medicines. The strategy relying on o-quinone methides has been used for the total synthesis of variety of natural products of this family like equol, 3'-hydroxyequol, vestitol and myristinins.
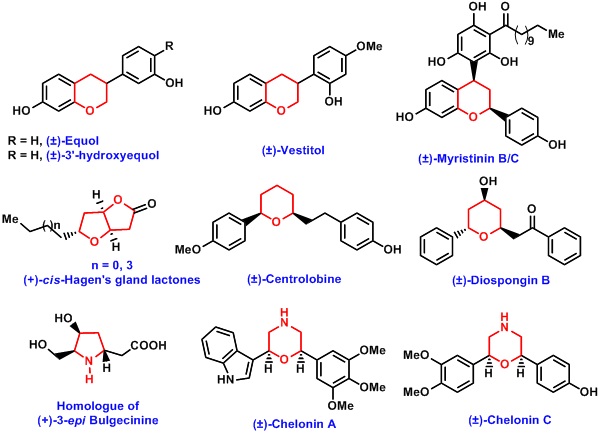
Very recently, we have also developed stereoselective strategies for construction of morpholines and oxazepenes, which are important pharmacophores.This method also used for the total synthesis of chelonin A and Chelonin C.
Research Highlights :
- "Shortest Route to Mucocin"
C & E News 2003, 81(49), 32. - "Synthesis of Heterocyclic Triquinanes via Sequential Radical Cyclizations"
Victor Snieckus and Nathan E. Genung (Pfizer), Synfacts 2013, 9, 33. - "Young Career Focus: Dr. Santosh J. Gharpure (Indian Institute of Technology Bombay, Mumbai, India)"
Synstories, SYNFORM, 2014/03, Published online: 17.02.2014, DOI: 10.1055/s-0033-1340792. - "Stereoselective Synthesis of Cyclic Ethers by Reductive Cyclization of Alkynols"
Victor Snieckus and Andy Tsai (Pfizer), Synfacts 2018, 14, 0245. - "Benzoxazoles and Benzoxazines by Intramolecular Aryl Azide Insertion"
Victor Snieckus and Andy Tsai (Pfizer), Synfacts 2019, 15, 0486.




